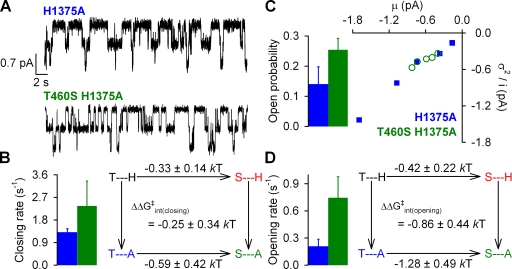
Figure 8.
Effects of mutations at positions 460 and 1375 on normal hydrolytic channel gating. (A) Representative single-channel current traces from prephosphorylated H1375A and T460S/H1375A CFTR channels gating in 2 mM ATP. Downward deflection indicates inward current. (B; left) Closing rates of H1375A (blue bar) and T460S/H1375A (green bar), defined as the inverse of the mean burst duration (see Materials and methods). (Right) Thermodynamic mutant cycle for target pair T460-H1375 built on closing rates. The top two corners of the mutant cycle (representing WT and T460S) were taken from Fig. 2 C. (C) Noise analysis was used to estimate Po for H1375A (blue bar) and T460S/H1375A (green bar). (D; left) Opening rates of H1375A (blue bar) and T460S/H1348A (green bar), obtained using the estimate for Po (see C) and closing rate (see B). (Right) Thermodynamic mutant cycle for target pair T460-H1375 built on opening rates. The top two corners of the mutant cycle were taken from Fig. 3 D.