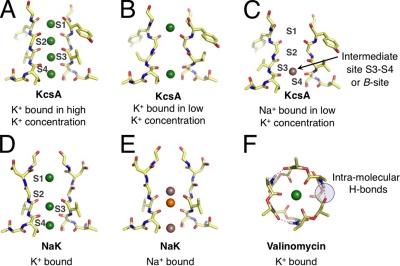
Figure 1.
Representative binding modes of Na+ and K+ ions in structural motifs of K+-selective membrane transport molecules. (Note that only two units from the tetrameric selectivity filters are shown for clarity.) (A) The selectivity filter of KcsA (Zhou et al., 2001) adopts different configurations under conditions of high and (B) low K+ concentrations, presenting K+ with different sets of binding modes. (C) Under rare conditions when Na+ binds to the KcsA filter, Na+ prefers a binding site different from K+ (Nimigean and Miller, 2002; Shrivastava et al., 2002; Lockless et al., 2007; Thompson et al., 2009). (D) The bacterial NaK channel, which belongs to the family of CNG channels, has a selectivity filter architecture similar to KcsA, but is only weakly selective for K+. Initially, low temperature x-ray data suggested binding modes for K+ that are identical to Na+. (E) Newer higher resolution crystallographic studies show more variety in Na+ binding, attributing electron density at the S3 site to competitive binding of a contaminant (orange) with Na+, with other Na+-binding sites between planes of carbonyl or hydroxyl oxygens (Alam and Jiang, 2009). (F) In comparison to KcsA and the NaK channel, the K+-selective bacterial toxin molecule (Dobler, 1981), valinomycin, binds K+ differently, using six (or fewer) carbonyl oxygens, instead of eight (or fewer) as in KcsA and NaK.