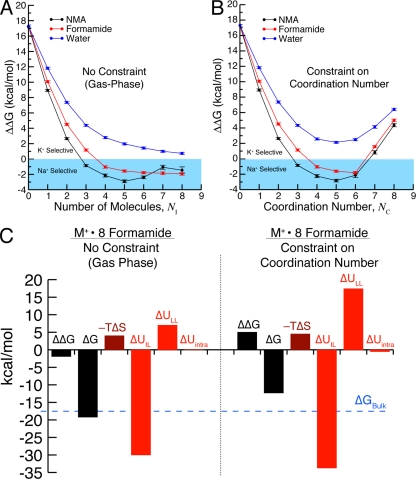
Figure 4.
Results from binding site models demonstrating the effect of an external field, in the form of an ion coordination number constraint, on K+/Na+ selectivity. Calculations were performed using the AMOEBA polarizable force field (Bostick and Brooks, 2010). (A) ΔΔG versus the number of included molecules, NI, in gas-phase clusters around K+ and Na+ in the absence of a constraint on coordination. As NI increases, the observed selectivity approaches values expected for bulk liquids (for water, ΔΔG ≈ 0; for formamide/NMA, ΔΔG < 0). Note that the number of included molecules, NI, is not necessarily equal to the number of coordinating molecules, NC, because of the absence of a coordination constraint. (B) ΔΔG versus the number of molecules, NC, directly coordinating K+ and Na+. In agreement with quantum mechanical calculations (Varma and Rempe, 2007, 2008), ΔΔG is larger in the water-based models (blue) than in the carbonyl-based models (black and red). Because of the presence of an external field (half-harmonic confinement) that imposes a specific coordination number, K+ selectivity is observed for seven or more ligands. In the models that coordinate K+ or Na+ with carbonyl ligands, K+ selectivity is determined by the external field rather than the ligand identity (NMA, formamide, and water) because the binding site models are Na+ selective in the absence of the constraint on coordination number, NC. (C) Contributions from specified individual components of the selectivity free energy (Eq. 2) in models composed of eight formamide molecules: ligand–ligand interactions, ion–ligand interactions, intramolecular interactions, and entropy In the case considered here, the contribution from the external field, is negligible. In the absence of the field (left), the components yield net Na+ selectivity (ΔΔG < 0). When a field enforces eightfold coordination, the distribution of these individual components changes, producing net K+ selectivity (ΔΔG > 0). Thus, the redistribution of the individual components, and therefore the net K+ selectivity, is an effect of the applied external field.