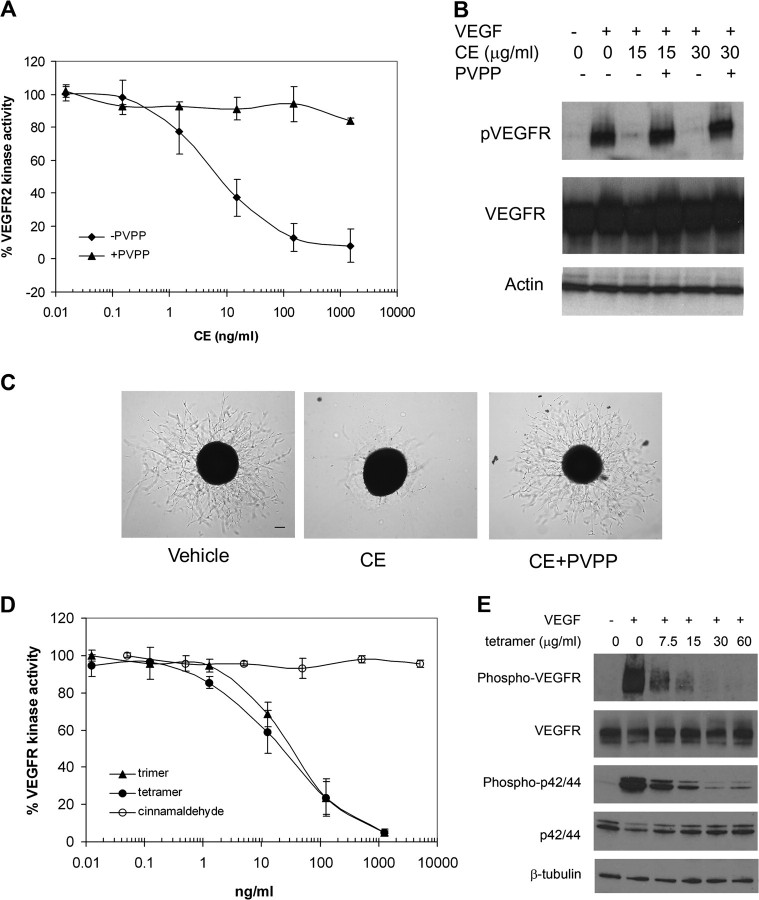
Fig. 6.
Oligomeric procynanidins inhibit VEGFR2 kinase activity. (A–C) PVPP treatment depletes anti-angiogenesis activity in CE. PVPP depletion of procynanidins from CE (see Materials and Methods) resulted in (A) inhibited kinase activity of purified VEGFR2, and data are mean ± SD (n = 3), (B) phosphorylation of VEGFR2 in HUVEC and (C) sprout formation from aortic ring. Scale bar, 100 μm. Experiments were repeated at least twice. (D) Oligomeric procyanidins suppressed kinase activity of purified VEGFR2. Cinnamaldehyde, trimer or tetramer of procyanidins, was incubated with VEGFR2. Data represent the percentage of kinase control (without CE treatment) and are mean ± SD (n = 3). (E) Oligomeric procyanidins inhibited VEGFR2 signaling. HUVECs were incubated in the presence or absence of trimer and tetramer of procyanidins, followed by VEGF stimulation. Phosphorylation of VEGFR-2 or MAPK was assessed by western blot using anti-phospho-VEGFR2 antibody, anti-phospho-MAPK antibody, as well as anti-total VEGFR2 and anti-total MAPK antibody. Experiments were repeated twice.