Abstract
Schizophrenia is a psychiatric disorder that causes significant disability and morbidity. Narcolepsy is a disorder, less prevalent than schizophrenia, but a disorder in which symptoms overlap with schizophrenia. This overlap in symptoms can cause narcolepsy to be confused with schizophrenia. The differences and similarities between narcolepsy and schizophrenia are discussed in the context of two cases. The first case describes an adolescent and the second case describes a refractory case of narcolepsy that did not respond to stimulants. Both cases were previously diagnosed as schizophrenia and did not respond to trials of antipsychotics and other psychotropics. The patients were reevaluated and referred to sleep testing at our facility for clinical suspicion of narcolepsy. Both patients underwent polysomnography with subsequent multiple sleep latency testing. The sleep testing results and multiple sleep latency testing criteria for narcolepsy are also discussed. The patients were treated for narcolepsy resulting in remission of the psychotic symptoms with significant behavioral improvement. We recommend that psychiatrists consider narcolepsy in the differential diagnosis when faced with refractory psychosis.
Keywords: narcolepsy, schizophrenia, hypersomnia, visual hallucinations, mean sleep latency testing (MSLT)
Introduction
Schizophrenia and narcolepsy share common symptoms and can be difficult to differentiate clinically. The spectrum of symptoms in both conditions can overlap leading to the misdiagnosis of narcolepsy as schizophrenia.1
Brief overview of schizophrenia. Schizophrenia is usually chronic and causes lifelong morbidity and significant disability. The prevalence of schizophrenia is approximately one percent in the general population, and there are no significant race or gender differences in the prevalence of the disease.2 Significant advances in identifying genetic abnormalities and marker have occurred.3 There is a strong hereditary link in schizophrenia, and having one parent with schizophrenia significantly increases the risk of the disease in children.4 Brain imaging studies have detected differences between the schizophrenic brain and healthy controls.5,6 However, the exact pathophysiology of the disease remains elusive. The hallmarks of schizophrenia are a disorganized behavior and speech and the presence of psychosis.7 Psychosis in schizophrenia includes hallucinations and delusions. Delusions are usually paranoid in nature but can also be religious, somatic, or bizarre. The most common type of hallucinations is auditory; however, visual and other sensory hallucinations can occur. Schizophrenia usually manifests in late adolescence in male individuals and in female individuals in the early to mid-20s.2 Treatment of schizophrenia is usually with antipsychotics. This class of medications mainly antagonizes dopamine receptors as well as modulating other neurotransmitters in the brain. In addition to dopamine antagonism, serotonergic, histaminergic, adrenergic, and cholinergic pathways are affected by antipsychotics.8 Antipsychotics and other psychotropics frequently used to control the symptoms of schizophrenia can cause sedation and somnolence. The disorganized thought process and behaviors of schizophrenia can lead to disruption of the sleep-wake cycle and frequently manifests as daytime somnolence. Schizophrenia is diagnosed by a formal psychiatric evaluation; currently, there are no specific objective diagnostic tests.2,7
Brief overview of narcolepsy. Narcolepsy causes unstable sleep and wake states. The sudden intrusion of sleep into wakefulness causes excessive sleepiness and introduces rapid eye movement (REM) sleep-related phenomena into wakefulness.9 REM-related phenomena include skeletal muscle atonia, autonomic instability, hypnogogic hallucinations, somnolence, and autonomic instability. Narcolepsy also causes the fragmentation of nocturnal sleep, and sleep becomes fragmented from frequent awakenings interrupting sleep.10 The prevalence of narcolepsy in the general population is 0.01 to 0.18 percent. Narcolepsy is more prevalent in Japan and less common in Israel and has a slight male preponderance. Narcolepsy usually manifests in the second decade of life.11 The underlying pathophysiology in narcolepsy is a loss of hypocretin-producing cells in the posterior hypothalamus. Narcolepsy is strongly associated with the human leukocyte antigen (HLA) DQB1*0602.12 A pentad of symptoms characterizes narcolepsy: hypersomnia, hypnogogic/hypnopompic hallucinations, sleep paralysis, cataplexy, and disturbed nocturnal sleep. Cataplexy occurs in the context of strong emotions, usually laughter, and can be subtle (a slight sagging of the jaw) or dramatic (generalized loss of muscle tone leading to falling). Consciousness is maintained during cataplexy. The hallucinations are vivid visual hallucinations from the intrusion of REM into wakefulness. They can be brief and startling or they can be complex and elaborate.13 However, other hallucinations can also occur in the context of narcolepsy. The hypersomnia and disrupted sleep-wake cycle in narcolepsy can lead to unusual behaviors and significant disability, which can be similar to the disorganized behaviors of schizophrenia. Unlike schizophrenia, there are specific tests available for narcolepsy. These include mean sleep latency testing (MSLT), HLA typing, and measuring hypocretin levels in cerebrospinal fluid.11–13
Testing. MSLT, a common diagnostic test for narcoleps, is an electrophysiological test to objectively evaluate hypersomnia. The test is performed after an overnight polysomnography (PSG). If the PSG does not show sleep-disordered breathing or a movement disorder that can account for the hypersomnia, then the MSLT is performed. MSLT is a series of five naps, two hours apart. The first nap is two hours after awakening. A nap is terminated after 20 minutes if no sleep occurs. If sleep occurs, the nap is terminated after 15 minutes. In between naps, patients are not allowed to use caffeine, stimulants, sedatives, or exercise. The diagnostic criteria for narcolepsy, as per the International Classification of Sleep Disorders, Second Edition (ICSD-2), are a mean sleep latency (MSL) of less than eight minutes in the five naps and at least two of the naps having sleep onset REM periods (SOREMPS).12 The Epworth Sleepiness Scale (ESS) measures hypersomnia, has a range from 0 to 24, and a score greater than 10 indicates excessive daytime sleepiness.14 The ESS can measure the degree of patient-reported hypersomnia and is a helpful tool for evaluating response to treatment. Central nervous system stimulants and wake-promoting agents are the main therapy for narcolepsy.15 Adjunct treatment, especially for cataplexy, includes selective serotonin reuptake inhibitors and serotonin norepinephrine reuptake inhibitors.16 Sodium oxybate (gamma aminobutyric acid [GABA] agonist) is indicated for narcolepsy with fragmented nocturnal sleep or refractory cataplexy.16,17
Case Presentations
We present two cases that reflect the clinical implications of the above discussion. Both patients were being treated for refractory schizophrenia. PSG and MSLT results from both cases are also discussed.
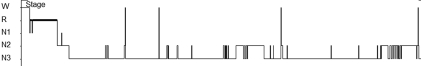
Case 1. An 18-year-old African American woman with schizophrenia and pervasive developmental disorder presented to our clinic for persistent hypersomnia. She reported sleeping 14 to 16 hours a day (nocturnal sleep was approximately 10–12 hours with 2–3 daytime naps 1–2 hours each). The hypersomnia caused significant impairment academically and socially. She was diagnosed two years previously with schizophrenia. The patient reported vivid visual hallucinations for at least two years. She did not report any auditory or other hallucinations. Paranoid or bizarre delusions were not present. No evidence of disorganized behavior or speech was noted. Visual hallucinations consisted of human figures in dark cloaks. The hallucinations were almost exclusively hypnogogic. The patient was obese, had a history of a corrected congenital cardiac malformation (ventricular septum defect), and was on digitalis. She was previously treated for almost one year with risperidone. The visual hallucinations did not remit, and she developed galactorrhea from the risperidone. She was subsequently switched to haloperidol. The galactorrhea resolved but the hallucinations persisted. Due to concerns about snoring and obesity, a PSG was ordered. MSLT was ordered for clinical suspicion of narcolepsy due to the severe hypersomnia, visual hallucination, and episodes of mild cataplexy. Cataplexy in this patient presented as transient sagging of the jaw and, occasionally, as head drooping due to neck muscle weakness. PSG findings were suggestive of possible narcolepsy. Those findings were decreased sleep onset latency (SOL) of nine minutes and sleep onset REM of one minute (Figure 1). Total sleep time (TST) of 473 minutes was within normal limits. Sleep architecture was not fragmented with a sleep efficiency index of 97 percent, an arousal index (AI) of 0.5/hour, and decreased REM at 6.7 percent of TST (Figure 1). The apnea hypopnea index (AHI), which measures the degree of sleep-disordered breathing, was 0.1 (AHI<5 is normal). There were no periodic leg movements detected. The MSLT confirmed the diagnosis of narcolepsy with mean sleep latency (MSL) of one minute and 12 seconds and five naps with SOREMPS. The patient was considered for centrally acting stimulants and wake-promoting agents. Modafanil was our first choice due to its favorable side effect profile, efficacy, and tolerability.14 Because of the cardiac history in this patient, a consultation and clearance to use stimulants was obtained from her cardiologist. Consent from the patient and her guardian was obtained after risks and benefits were explained. She was started on modafanil 200mg daily. At the two-week follow up, the hallucinations had resolved and the hypersomnia improved. Night time sleep became consolidated, and the patient maintained wakefulness in the daytime. The ESS improved from 21 (severe) pre-treatment to 8 (normal) post-treatment. No adverse effects were reported. The patient at the two-month follow up was doing well. Haloperidol reduction in this patient was well tolerated, and the long-term goal is to discontinue antipsychotics.
Figure 1.
Histogram from Case 1 showing decreased sleep onset latency and sleep onse: R=REM; N1=stage 1 sleep; N2=stage 2 sleep; N3=stage 3 sleep
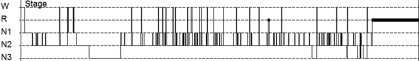
Case 2. A 41-year-old Caucasian woman with a diagnosis of schizophrenia was referred to our clinic to be evaluated for hypersomnia. She reported chronic depression and visual hallucinations. There were no auditory hallucinations reported by this patient and no delusions. She reported sleepiness since she was a child. Routinely, she slept 7 to 8 hours per night and complained of severe daytime hypersomnia. The patient frequently napped 1 to 2 hours during the day. She was unable to maintain a job and would frequently fall asleep during the work day. She subsequently became very depressed and developed suicidal ideation. This patient had no history of substance abuse. The visual hallucinations did not respond to multiple trials of typical and atypical antipsychotics. She reported snoring and unrefreshing sleep. There was no history suggestive of cataplexy. Her medical history included diabetes, hypertension, hypothyroidism, and headaches. Medications included thyroxine, carbamazepine, diazepam, haloperidol, quetiapine, metoprolol, venlafaxine, eletriptan, metformin, and glipizide. PSG and MSLT were ordered. PSG was suggestive of narcolepsy with a decreased SOL of five minutes and an increased arousal index (AI) of 40 arousals per hour, indicative of fragmented sleep (AI<10 is normal). TST of 498 minutes was within normal limits. The AHI was 1.2 (normal), and moderate snoring was reported. No periodic leg movements were detected. MSLT confirmed the diagnosis of narcolepsy with MSL of five minutes and 54 seconds and three SOREMPS. After narcolepsy was confirmed, modafanil 200mg daily was started. There was only a minimal improvement reported. After two weeks, she was switched to amphetamine salt 20mg daily. She was unable to tolerate the side effects of anxiety and agitation. Due to the high degree of sleep fragmentation noted on her PSG (Figure 2) and the severity of the hypersomnia, she was started on sodium oxybate. Sodium oxybate was initiated at a nightly dose of 4.5g orally (supplied in a 0.5g/mL solution). The dose was divided equally with 2.25g at bedtime and 2.25g three hours after bedtime. She reported improvement in daytime hypersomnia and a resolution of the visual hallucinations. Nausea was reported as a side effect that improved after one week. ESS improved from 15 (severe) pre-treatment to 9 (normal) post-treatment. The long-term goal is to taper and reduce psychotropic medications.
Figure 2.
Histogram from Case 2 showing decreased sleep onset latency and sleep fragmentation: R=REM; N1=stage 1 sleep; N2=stage 2 sleep; N3=stage 3 sleep
Discussion
Both cases had findings on PSG (which preceded the MSLT) suggestive of narcolepsy. The first case had decreased SOL and sleep onset REM (Figure 1), and the second case had decreased SOL and increased sleep fragmentation (Figure 2). In both hypnograms, sleep architecture indicates reduced REM sleep. In Case 1, REM was 6.7 percent of TST and in Case 2, REM was 12 percent of TST. Decreased REM sleep in both cases was most likely multifactorial due to the REM-reducing effects of the psychotropics both patients were receiving and from REM fragmentation of narcolepsy. Complicating psychiatric factors were present in both patients. The disorganized behavior from hypersomnia and visual hallucinations likely facilitated the diagnosis of schizophrenia. A large review that compared psychotic symptoms in schizophrenia and narcolepsy found that the most useful distinguishing features are visual hallucinations in narcolepsy versus mainly auditory hallucinations in schizophrenia, and the delusional component is absent in psychosis of narcolepsy.18 There are reports in the literature of narcolepsy misdiagnosed as schizophrenia.19–22 These cases were refractory to standard schizophrenia therapies and when reevaluated led to a diagnosis of narcolepsy. The adolescent case presented here was similar to reports on childhood narcolepsy.23 These reports are a reflection of the increasing awareness among physicians that narcolepsy can present as schizophrenia.
Conclusion
The treating physician should consider narcolepsy in the differential of unusual or refractory schizophrenia. Narcolepsy can be confirmed by safe and noninvasive testing, mainly the MSLT. Treatment for narcolepsy is safe and effective. In certain cases, when hypersomnia or symptoms of narcolepsy recur or are exacerbated, a repeat MSLT or maintenance of wakefulness test can be considered to reevaluate the efficacy of treatment or the need for adjusting or modifying the stimulant and waking-promoting agents. However, as in the cases discussed here, there are cases that do not respond to standard stimulant or wake-promoting agent therapy. Some patients can have comorbidities that require special attention when using stimulants. The co-occurrence of narcolepsy and schizophrenia in the same patient is unlikely.24
References
- 1.Dahmen N, Kasten K, Muller MJ. Narcoleptic and schizophrenic hallucinations. Implications for differential diagnosis and pathophysiology. Eur J Health Econ. 2002;3(Suppl 2):S94–98. doi: 10.1007/s10198-002-0113-x. [DOI] [PubMed] [Google Scholar]
- 2.Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia: “just the facts”—what we know in 2008: epidemiology and etiology. Schizophr Res. 2008;102(1-3):1–18. doi: 10.1016/j.schres.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Atz ME, Rolllins B, Vawter MP. NCAM1 association study of bipolar and schizophrenia: polymorphisms and alternatively spiced isoforms lead to similarities and differences. Psychiatr Genet. 2007;17(2):55–67. doi: 10.1097/YPG.0b013e328012d850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Liu J, Feng G, et al. The MDGA1 gene confers risk to schizophrenia and bipolar disorder. Schizophr Res. 2010 doi: 10.1016/j.schres.2010.11.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Goghari VM, Sponheim SR, Macdonald AW., 3rd The functional neuroanatomy of symptom dimensions in schizophrenia: a qualitative and quantitative of a persistent question. Neurosci Biobehav Rev. 2010;34(3):468–486. doi: 10.1016/j.neubiorev.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyer P, Phillips JL, Rousseau FL, Ilivitsky S. Hippocampal abnormalities and memory deficits: new evidence of a strong pathophysiological link in schizophrenia. Brain Res Rev. 2007;54(1):92–112. doi: 10.1016/j.brainresrev.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 7.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition, Text Revision. Washington, DC: American Psychiatric Press Inc.; 2000. [Google Scholar]
- 8.Gardner DM, Baldessarini RJ, Waraich P. Modern antipsychotic drugs: a critical overview. CMAJ. 2005;172(13):1703–1711. doi: 10.1503/cmaj.1041064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benca RM. Narcolepsy and excessive daytime sleepiness: diagnostic considerations, epidemiology, and comorbidities. J Clin Psychiatry. 2007;8(Suppl 13):5–8. [PubMed] [Google Scholar]
- 10.Piazzi G, Serra L, Ferri R. Nocturnal aspects of narcolepsy with cataplexy. Sleep Med Rev. 2008;12(2):109–128. doi: 10.1016/j.smrv.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Nishino S. Clinical and neurobiological aspects of narcolepsy. Sleep Med. 2007;8(4):373–399. doi: 10.1016/j.sleep.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Academy of Sleep Medicine. Diagnostic and Coding Manual. Second Edition. Westchester, IL: American Academy of Sleep Medicine; 2005. International Classification of Sleep Disorders. [Google Scholar]
- 13.Nishino S. Hypocretin/orexin and narcolepsy: new basic and clinical insights. Acta Physiol. 2010;198(3):209–222. doi: 10.1111/j.1748-1716.2009.02012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376–381. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 15.Kumar R. Approved and investigational uses of modafanil: an evidence based review. Drugs. 2008;68(13):1803–1839. doi: 10.2165/00003495-200868130-00003. [DOI] [PubMed] [Google Scholar]
- 16.Zaharna M, Dimitriu A, Guilleminault C. Expert opinion on pharmacotherapy of narcolepsy. Expert Opin Pharmacother. 2010;10:1633–1645. doi: 10.1517/14656566.2010.484021. [DOI] [PubMed] [Google Scholar]
- 17.Kantrowitz JT, Citrome L, Javitt DC. A review of the tolerability and abuse liability of gamma-hydroxybutyric acid for insomnia in patients with schizophrenia. Clin Ther. 2009;31(Pt1):1360–1373. doi: 10.1016/j.clinthera.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Fortuyn HA, Lappenschaar GA, Nienhuis FJ, et al. Psychotic symptoms in narcolepsy: phenomenology and a comparison with schizophrenia. Gen Hosp Psychiatry. 2009;31(2):146–154. doi: 10.1016/j.genhosppsych.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Szucs A, Janszky J, Hollo A, Migleczi G, Halasz P. Misleading hallucinations in unrecognized narcolepsy. Acta Psychiatr Scand. 2003;108(4):314–316. doi: 10.1034/j.1600-0447.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 20.Walterfang M, Upjohn E, Velakoulis D. Is schizophrenia associated with narcolepsy? Cogn Behav Neurol. 2005;18(2):113–118. doi: 10.1097/01.wnn.0000160822.53577.2c. [DOI] [PubMed] [Google Scholar]
- 21.Undurraga J, Garrido J, Santamarí J, Parellada E. Treatment of narcolepsy complicated by psychotic symptoms. Psychosomatics. 2009;50(4):427–428. doi: 10.1176/appi.psy.50.4.427. [DOI] [PubMed] [Google Scholar]
- 22.Bhat SK, Galang R. Narcolepsy presenting as schizophrenia. Am J Psychiatry. 2002;159(7):1245. doi: 10.1176/appi.ajp.159.7.1245. [DOI] [PubMed] [Google Scholar]
- 23.Nevsimalova S. Narcolepsy in childhood. Sleep Med Rev. 2009;13(2):169–180. doi: 10.1016/j.smrv.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Kishi Y, Konishi S, Kudo Y, Kurosawa H, Kathrol RG. Schizophrenia and narcolepsy: a review with a case report. Psychiatry Clin Neurosci. 2004;58(2):117–124. doi: 10.1111/j.1440-1819.2003.01204.x. [DOI] [PubMed] [Google Scholar]