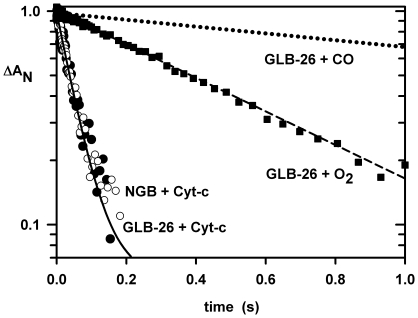
Figure 4. Electron transfer kinetics.
Electron transfer from GLB-26 at 25°C. In parallel to the reversible CO and His binding (415 nm), additional shunting reactions were probed in the presence of Cyt-c (415 nm) or oxygen (410 nm). These reactions were initiated by photolysis of the CO form of 2 µM GLB-26. The pentacoordinated photoproduct has a lifetime of only 50 µs, so ms reactions shown here concern the His-Fe-His intermediate state. The top curve (dotted line) is for samples with only CO, which requires several seconds for the His to CO replacement reaction. In the presence of 2 µM Cyt-c, an electron transfer reaction occurs in a tenth of a second (black circles). A similar rate was observed for human Ngb (white circles). GLB-26 was equilibrated under CO (1 atm) and then diluted in a deoxygenated buffer containing Cyt-c (final [CO] was only a few µM); under this condition the His rebinding is much faster than that of CO allowing the formation of a bis-histidine hexacoordinated heme which transfers an electron to Cyt-c. In the presence of oxygen 1/3 atm and CO 2/3 atm (without Cyt-c) GLB-26 is oxidized in less time than the His dissociation, indicating an oxidation of the Fe-His complex without oxygen binding to the iron atom (black squares).