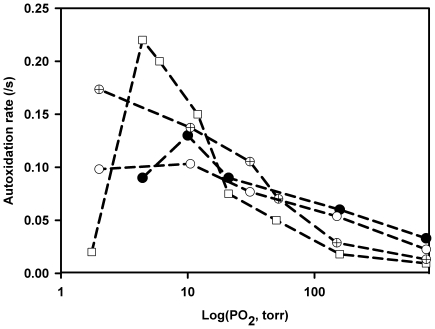
Figure 6. Rate of autoxidation versus PO2.
Human Ngb WT (crossed circles), Ngb WT without disulfide bridge after reduction with DTT (white circles), human Ngb triple cysteine mutant Cys(CD7)→ Gly, Cys(D5)→ Ser and Cys(G19)→ Ser (black circles) and human Cygb double mutant Cys (B2)→ Ser and Cys (E9) → Ser (white squares). The acceleration of the rate of autoxidation for PO2 close to the P50O2 is assumed to be due to a bimolecular reaction between the ferrous hexacoordinated form and the molecular O2 while at high PO2 the dominant reaction is the unimolecular oxidation after O2 binding to the heme. Experimental conditions were: 25 mM potassium phosphate buffer, 0.1 mM EDTA, 37°C. Protein concentrations were between 2 and 4 µM.