Abstract
Barrett's esophagus has gained increased clinical attention because of its association with esophageal adenocarcinoma, a cancer with increasing incidence and poor survival rates. The goals of ablating Barrett's esophagus are to decrease esophageal cancer rates and to improve overall survival and quality of life. Different techniques have been developed and tested for their effectiveness eradicating Barrett's epithelium. This review assesses the literature associated with different ablative techniques. The safety and efficacy of different techniques are discussed. This review concludes with recommendations for the clinician, including specific strategies for patient care decisions for patients with Barrett's esophagus with varying degrees of dysplasia.
Keywords: Ablation, Argon plasma coagulation, Barrett's esophagus, Cryotherapy, Dysplasia, Endoscopy, Endoscopic mucosal resection, Esophageal adenocarcinoma, Photodynamic therapy, Proton pump inhibitor, Radiofrequency ablation
Introduction
Ablative therapy for Barrett's esophagus (BE) aims to destroy the abnormal columnar epithelium, allowing for replacement of the esophageal mucosa with neosquamous epithelium. Over the past several years, different techniques have been developed to ablate BE. The purpose of this review is to synthesize data in support of the various ablative therapies, and to compile and discuss known risks and benefits associated with each. Finally, we provide recommendations regarding applications of ablative therapy for BE in clinical practice, and propose future research questions for studying ablation of BE.
Why Ablate Barrett's Esophagus?
BE represents a metaplastic change in the esophagus, in which normal squamous epithelium is replaced with columnar epithelium. Because BE is the premalignant condition associated with esophageal adenocarcinoma, much of the urgency and fear regarding BE is linked to the abysmal mortality of esophageal adenocarcinoma (10% to 15% 5-year survival) [1–3]. The increasing incidence of esophageal cancer over the past few decades—as much as 463% in white men [4]—is concerning and still poorly understood.
Although the incidence of esophageal adenocarcinoma has increased at an alarming rate, the actual incidence remains relatively low, at 5.69 (95% CI, 5.47–5.91) per 100,000 [4]. By contrast, BE is a much more common condition. Because BE itself does not cause symptoms, the incidence and prevalence are difficult to estimate, but the prevalence of BE may be as high as 5 to 7% in the adult US population [5, 6]. The risk of developing esophageal adenocarcinoma in a person with known BE without dysplasia is estimated at 0.5% per patient-year [7, 8]. The vast majority of patients with BE will never develop cancer.
Barrett's Esophagus with High-Grade Dysplasia
In patients with BE and dysplasia, cancer risk increases. In patients with high-grade dysplasia, the risk of cancer progression may be as high as 19% per year [9•, 10]. In reports from the surgical literature, early esophageal adenocarcinoma is found incidentally in patients undergoing esophagectomy for high-grade dysplasia 40% of the time [11]. However, more recent data suggest that the rate of invasive carcinoma in the setting of high-grade dysplasia is 3% [11]. In addition to the mortality risk, patients with dysplastic Barrett's esophagus experience a decreased quality of life and experience anxiety that cancer will develop [12, 13•].
Because of the risk of cancer associated with high-grade dysplasia in BE, patients with high-grade dysplasia have traditionally been offered esophagectomy versus intense endoscopic surveillance at 3-month intervals. Esophagectomy is associated with significant morbidity and some mortality [14], and a substantial proportion of those suffering from high-grade dysplasia are poor surgical candidates. In patients with high-grade dysplasia, ablation of BE has already become widely practiced. However, long-term data showing mortality benefit with ablation are lacking.
Barrett's Esophagus with Low-Grade Dysplasia and Nondysplastic Barrett's Esophagus
For nondysplastic BE and for BE with low-grade dysplasia, the best approach to management of these conditions is less clear. The risk of progression to cancer in patients with low-grade dysplasia was recently estimated at 0.7% per year [15], but may be higher when the histological interpretation is confirmed by an expert pathologist [16]. Because cancer risk diminishes when comparing high-grade to low-grade and no dysplasia, other factors, such as the intervention's cost and side-effect profile, become more important in treatment decisions.
Adding complexity to the situation, the pathologic diagnosis of low-grade dysplasia in BE is notoriously difficult to make. In general for BE, interobserver and intraobserver reliability between pathologists was estimated at with kappa values of 0.43 to 0.46 (moderate) and 0.64 to 0.68 (good), respectively [17]. Although interobserver reproducibility for high-grade dysplasia and carcinoma was 0.64, for low-grade dysplasia, it was only fair at 0.32 [17]. One recent report of six hospitals without university affiliation in Amsterdam evaluated 147 patients who had been diagnosed with low-grade dysplasia. After a consensus review by expert pathologists, 85% of them were downgraded to nondysplastic BE [16]. In a report of 165 veterans with BE who underwent a subsequent surveillance endoscopy, 35.2% of patients were given a lower pathologic grade on repeat endoscopy and 11.5% progressed to a higher grade; the majority of patients with a change in degree of dysplasia were from the group with low-grade dysplasia [18]. This tendency to downgrading is less likely to represent natural regression, and more likely to represent sampling differences or misclassification by pathologists, particularly in the setting of inflammation. Problems establishing a true diagnosis of dysplasia, particularly in the community setting, make creating a surveillance and treatment plan for these patients difficult.
In clinical practice, subjects with nondysplastic BE and low-grade dysplasia are often enrolled in surveillance endoscopy programs. It is important to note that the evidence for this practice is not based on mortality data [19, 20]. The 2008 American College of Gastroenterology clinical guidelines rely upon cohort and case-control studies to support the recommendations of surveillance and management of dysplasia [19]. Although ablation has been demonstrated to cause reversion of epithelium to neosquamous in these subjects, and therefore, cosmetically at least, seems preferable to surveillance, further benefit must accrue to subjects to make it worth considering in subjects with lesser forms of dysplasia. Short-term data [9•] as well as decision analyses [15] suggest that there may be benefit in treating those with less advanced disease, but the field anxiously awaits further data.
Recent opinion articles and editorials have argued both that all BE should be ablated [21] and the opposing view that it is premature to ablate all BE in all patients [22]. What is agreed is that prospective, randomized, controlled trials of ablation of nondysplastic BE are lacking. Perhaps the single most important unanswered question in ablative therapies for BE is that of patient selection. Given the lack of conclusive data, it is difficult to be dogmatic in these decisions. A shared decision-making model, with frank discussion of what we do and do not know regarding these procedures, is essential.
The remainder of this review focuses on the available evidence regarding the efficacy and side effect profile of the various ablative strategies for BE.
Options for Barrett's Esophagus Ablation
Several different modalities have been used to ablate BE. These treatments include argon plasma coagulation, electrocoagulation, photodynamic therapy, BE eradication with endoscopic mucosal resection, cryotherapy, and radiofrequency ablation. All aim to remove or destroy the BE mucosa, allowing formation of a neosquamous epithelium.
Several factors must be considered in evaluating these ablative strategies. Strength of trial design should be noted, with randomized, controlled trials offering the best evidence. Overall improvement in cancer risk and mortality is the most important outcome, yet is rarely studied. A common theme in the literature on ablation of BE is relatively short duration of follow-up. Ease of use, patient safety, effectiveness ablating the BE mucosa, and the potential for “buried glands” (BE epithelium underneath the neosquamous epithelium) are more commonly reported than long-term outcomes such as survival.
A recent Cochrane review of published reports of BE treatments through 2008 provides a foundation from which to evaluate the data underlying ablative therapy in BE [23]. This review encompasses the 11 randomized trials of ablative therapy for BE performed through 2008. In addition to these randomized trials, many case series have been published that provide additional information about ablative therapy. The weakness of a case series in general is lack of randomization and lack of a control group. However, these studies can be particularly helpful at evaluating complication rates, and may be useful for generating data regarding long-term durability of response.
In this section, we review the basic information regarding the various types of ablative therapy, with an emphasis on new and recent data. Response rates to ablation techniques as reported in the literature are given in Table 1. Complications associated with different modes of ablation and findings of buried glands are reported in Table 2.
Table 1.
Histologic response rates reported for studies of ablation of intestinal metaplasia and dysplasia in Barrett's esophagus
| Study | Sample size/type of dysplasia | Length of follow-up | Complete histologic IM eradication at first reported follow-up (%) | Complete eradication dysplasia (%) | Persistence of IM eradication after initial response (note that times to follow-up vary) | Eradication of all BE at end of study (%) |
|---|---|---|---|---|---|---|
| MPEC | ||||||
| Sampliner et al. [25] | 58 NDBE | 6 mo | 78% | n/a | n/a | n/a |
| APC | ||||||
| Bright et al. [29] | 40 no HGD 20 APC 20 surveillance |
68 mo mean | n/a | n/a | n/a | At 5 y follow-up 40% (8/20) APC group had complete regression vs 20% (4/20) surveillance group |
| PDT | ||||||
| Ackroyd et al. [32] | 36 LGD 18 PDT 18 placebo |
24 mo | No complete eradications (by design) | 100% PDT in treated area 33% placebo |
n/a | n/a |
| Overholt et al. [10, 33] | 208 HGD 138 PDT 70 omeprazole |
24 mo and then 5 y | 52% PDT 7% omeprazole |
For all dysplasia: 59% PDT and 4% omeprazole |
n/a | n/a |
| For HGD: 77% PDT 39% omeprazole |
||||||
| Badreddine et al. [34] | 363: 12 NDBE, 56 LGD, 190 HGD, 105 intramucosal carcinoma (EMR used with PDT in some) | 36 mo median | 48% overall (175/363) | 72% (261/363) with reduction in dysplasia after PDT (unknown how many had complete eradication of dysplasia) | Number with recurrent BE unknown; 83% without return of dysplasia | n/a |
| APC vs Electrocoagulation | ||||||
| Dulai et al. [27] | 52: 51 NDBE, 1 LGD 26 MPEC 26 APC |
Unknown | 81% MPEC 65% APC |
n/a | n/a | n/a |
| Sharma et al. [28] | 35: 32 NDBE, 3 LGD 16 MPEC 19 APC |
2 y minimum | Complete response 83% for both groups | n/a | 83% (24/29) | After 2 y, 24/35 (68%) of patients had complete response (75% [12/16] MPEC, 63% ([12/19] APC) |
| PDT vs APC | ||||||
| Hage et al. [35] | 40: 32 NDBE, 8 LGD 13 PDT single dose 12 PDT fractionated doses 14 APC |
18 mo | PDT single dose 8% PDT fractionated doses 33% APC 36% |
n/a | n/a | PDT single dose 75% PDT fractionated doses 88% APC 67% (Some received additional APC following initial study treatment) |
| Kelty et al. [36] | 68 NDBE 34 PDT 34 APC |
12 mo median | PDT 38% APC 77% |
n/a | n/a | n/a |
| RFA | ||||||
| Roorda et al. [40] | 13: 6 NDBE, 4 LGD, 3 HGD | 12 mo mean | 46% (6/13) | 71% (5/7) | n/a | n/a |
| Sharma et al. [37], Fleischer et al. [38, 39] | 32 in dosimetry and 70 in effectiveness all NDBE | 2.5 y and 5 y | 59% (19/32) dosimetry 69% (48/70) effectiveness at 12 mo |
n/a | 2.5 y: 97% (60/62) 59 patients were treated with focal ablation during the follow-up period 5 y: 92% (46/50) |
2.5 y: 86% (60/70) 59 patients were treated with focal ablation during the follow-up period 5 y: 66% (46/70) |
| Gondrie et al. [41] | 11: 2 LGD, 9 HGD | 14 mo median | 100% | 100% | n/a | n/a |
| Gondrie et al. [42] | 12: 11 HGD, 1 LGD | 14 mo median | 100% | 100% | n/a | n/a |
| Sharma et al. [43] | 10 LGD | 24 mo | 70% at 1 year | 90% at 1 year | n/a | 90% at 2 y (after additional ablations) |
| Hernandez et al. [44] | 10: 7 NDBE, 2 LGD, 1 HGD | 12 mo | 70% | n/a | n/a | n/a |
| Ganz et al. [46] | 142 HGD | 12 mo median | 54% | 80% | n/a | n/a |
| Sharma et al. [45] | 63: 39 LGD, 24 HGD | 24 mo median | 79% | 89% | n/a | n/a |
| Eldaif et al. [56] | 27: 22 NDBE, 2 LGD | 0 | 100% | n/a | n/a | n/a |
| Shaheen et al. [9] | 127: 64 with LGD and 63 with HGD 84 RFA 43 sham procedure |
12 mo | 77% RFA 2% sham control |
90% RFA 23% sham control |
n/a | n/a |
| Velanovich et al. [48] | 66: 12 HGD | ? | 59% | n/a | n/a | 93% at 1 y (additional ablations between assessments) |
| Pouw et al. [47] | 24 23 underwent endoscopic resection first |
0 | 88% | 95% | n/a | n/a |
| Vassiliou et al. [49] | 15 with follow-up biopsies: 1 NDBE, 6 LDG, 15 HGD, 3 intramucosal carcinoma | 20 mo median | 73% | ? | n/a | n/a |
| Cryospray | ||||||
| Johnston et al. [50] | 11: varying degrees dysplasia | 6 mo | 78% | 100% | n/a | n/a |
| Dumot et al. [51] | 31 HGD or intramucosal adenocarcinoma | 12 mo median | 16% | 33% | n/a | 3% |
| Shaheen et al. [57] | 60 HGD (many had received previous therapy) | 10.5 mo mean | 57% | 97% | n/a | n/a |
| EMR | ||||||
| Chennat et al. [31] | 49 HGD and intramucosal carcinoma; 32 completed protocol | 17 mo median | 100% of the 32 who completed protocol 65% intention to treat | 100% of the 32 who completed protocol 65% intention to treat | 97% | 63% intention to treat |
| Pouw et al. [54] | 169 high-grade intraepithelial neoplasia or early cancer | 32 mo median | 89% | 99% (all neoplasia eradicated) | 88% | 81% |
APC—argon plasma coagulation; BE—Barrett's esophagus; EMR—endoscopic mucosal resection; HGD—high-grade dysplasia; IM—intestinal metaplasia; LGD—low-grade dysplasia; MPEC—multipolar electrocoagulation; n/a—not available; NDBE—nondysplastic Barrett's esophagus; PDT—photodynamic therapy; RFA—radiofrequency ablation
Table 2.
Complications as reported by study including overall complication rate, specific adverse events, and buried glands. Note that methods for collecting information on adverse events varied widely between studies
| Study | Total complications/number of patients | Pain | Dysphagia | Fever | Bleeding | Perforation | Stricture | Photosensitivity | Abnormal LFT | Nausea/vomiting | Buried glands |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MPEC | |||||||||||
| Sampliner et al. [25] | 25/58 MPEC | 33% | n/a | 3% | 2% | 0 | 2% | n/a | n/a | 1% | 5% |
| APC | |||||||||||
| Bright et al. [29] | 2/20 APC | n/a | n/a | n/a | n/a | n/a | 10% | n/a | n/a | n/a | 5% pre-treatment 10% surveillance 10% APC |
| PDT | |||||||||||
| Ackroyd et al. [32] | 19/18 PDT 0/18 placebo |
100% PDT | n/a | n/a | n/a | n/a | n/a | 6% | n/a | n/a | 0 |
| Overholt et al. [10, 33] | 132/133 PDT 51/69 PPI |
20% PDT | 19% PDT | 20% PDT | 0 | 1% during dilation | 36% PDT 0 PPI |
69% PDT (n=92) one with permanent scars |
n/a | 32% PDT | n/a |
| APC vs MPEC | |||||||||||
| Dulai et al. [27] | 1/26 APC 0/26 MPEC |
4% APC | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 0 when serial sections evaluated |
| Sharma et al. [28] | 17/19 APC 20/16 MPEC |
21% APC 38% MPEC |
11% APC 31% MPEC |
5% APC | 0 | 0 | 5% APC | n/a | n/a | n/a | n/a |
| PDT vs APC | |||||||||||
| Hage et al. [35] | 60/26 PDT 14/14 APC |
92% PDT 86% APC |
n/a | 31% PDT 14% APC |
n/a | n/a | n/a | n/a | 77% PDT 0 APC |
27% PDT 0 APC |
78% APC |
| Kelty et al. [36] | 23/34 PDT 67/34 APC |
3% PDT 100% APC |
94% APC | n/a | n/a | 0 | 3% APC | 15% PDT | 12% PDT | 32% PDT | 24% PDT 21% APC |
| RFA | |||||||||||
| Roorda et al. [40] | 6/13 RFA | 23% | 23% | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 0 |
| Sharma et al. [37], Fleischer et al. [38, 39] | 12/32 safety RFA 24/70 efficacy RFA |
12% 13% |
n/a | 9% 3% |
1% | n/a | n/a | n/a | n/a | 3% 11% |
0 |
| Gondrie et al. [41] | 12/11 RFA | 73% | 27% | 9% | 0 | 0 | 0 | n/a | n/a | n/a | 0 |
| Gondrie et al. [42] | 1/12 RFA | n/a | n/a | n/a | n/a | n/a | 8% | n/a | n/a | n/a | 0 |
| Sharma et al. [43] | 1/10 RFA | n/a | n/a | n/a | 10% | n/a | 0 | n/a | n/a | n/a | 0 |
| Hernandez et al. [44] | n/a RFA | Yes, but number not provided | n/a | n/a | n/a | 0 | 0 | n/a | n/a | n/a | 10% |
| Ganz et al. 2008 [46] | 1/142 RFA | n/a | n/a | n/a | n/a | n/a | 1% | n/a | n/a | n/a | 0 |
| Sharma et al. [45] | 2/63 RFA | n/a | n/a | n/a | 1% | n/a | 1% | n/a | n/a | n/a | 0 |
| Eldaif et al. [56] | 0/27 RFA | n/a | 0 | n/a | n/a | n/a | 0 | n/a | n/a | n/a | 0 |
| Shaheen et al. [9] | 8/84 RFA | 2% RFA | n/a | n/a | n/a | n/a | 6% RFA | n/a | n/a | 1% | 5% RFA 40% sham control |
| Velanovich et al. [48] | 4/66 RFA | n/a | n/a | n/a | n/a | n/a | 7% | n/a | n/a | n/a | n/a |
| Pouw et al. [47] | 9/24 RFA | n/a | 4% | n/a | 4% | 4% | 4% | n/a | n/a | n/a | 0 |
| Vassiliou et al. [49] | 5/59 RFA | n/a | n/a | n/a | 2% | n/a | 3% | n/a | n/a | 3% | 0 |
| Cryotherapy | |||||||||||
| Johnston et al. [50] | 4/11 cryotherapy | 18% | 18% | n/a | 0 | 0 | 0 | n/a | n/a | n/a | 18% |
| Dumot et al. [51] | 12/31 cryotherapy | 23% | 0 | n/a | n/a | 3% | 10% | n/a | n/a | 0 | n/a |
| Shaheen et al. [57] | 6/98 cryotherapy | 2% | n/a | n/a | 1% | n/a | 3% | n/a | n/a | n/a | 3% |
| EMR | |||||||||||
| Chennat et al. [31] | 20/49 EMR | 2% | n/a | n/a | 0 | 0 | 37% | n/a | n/a | n/a | 3% |
| Pouw et al. [54] | 91/169 EMR | n/a | n/a | n/a | 2% | 2% | 50% | n/a | n/a | n/a | 3% |
APC—argon plasma coagulation; EMR—endoscopic mucosal resection; LFT—liver function tests; MPEC—multipolar electrocoagulation; n/a—not available; PDT—photodynamic therapy; RFA—radiofrequency ablation.
Multipolar Electrocoagulation
Multipolar electrocoagulation was one of the early techniques used for ablation of BE [24]. In patients with nondysplastic BE, 6 months after a mean 3.5 ablation sessions, 78% of patients had no histologic evidence of BE [25]. Adverse events occurred in 43% of patients; chest pain and odynophagia were the most common symptoms associated with the treatment, although fever, gastrointestinal bleeding, and stricture also occurred [25]. Only a small amount of esophageal mucosa can be treated at a time, and multiple procedures were required to achieve ablation. The importance of performing surveillance endoscopy after ablation was established in these early studies as investigators learned that buried glands of BE were sometimes detected beneath normal-appearing squamous epithelium [26].
Argon Plasma Coagulation
Argon plasma coagulation (APC) has the ability to ablate BE successfully in about 70% of patients with BE without high-grade dysplasia (Table 1) [27, 28]. The major complications associated with argon plasma are pain and dysphagia, although strictures can occur at a rate of 5% to 10% (Table 2) [28, 29].
Over time, concern has emerged regarding APC and the presence of buried glands [30]. These buried glands may be more common after APC compared with other therapies (Table 2) [29].
APC is easy to use, with a reasonable safety profile. Providers have used it for ablating residual islands or tongues of BE, but this should be done with caution, given the risk of buried glands and reports of persistent disease after APC [31].
Photodynamic Therapy
Photodynamic therapy (PDT) uses an oral sensitizing medication, such as 5-aminolaevulinic acid or sodium porfimer, followed by exposure to light in the esophagus to promote damage to light-exposed cells. An early study of 36 patients with BE with low-grade dysplasia compared treatment with 5-aminolaevulinic acid PDT to a group who received light exposure to the esophagus alone without the sensitizing agent. In the treatment arm, 16 of 18 patients developed neosquamous epithelium in the treated area, whereas none of the patients in the control arm experienced conversion of the BE to neosquamous mucosa [32].
In a larger, multicenter, randomized, controlled trial of patients with BE with high-grade dysplasia that enrolled 208 patients at 30 sites, 138 patients were treated with PDT using porfimer sodium and 70 patients were treated with a proton pump inhibitor alone [10]. In this trial, 52% of those treated with PDT had total eradication of BE. The primary outcome of this trial, disappearance of all high-grade dysplasia in biopsies at any time after enrollment, was achieved in 77% of patients treated with PDT versus 39% of the control arm (P<0.0001). This response did not persist indefinitely. After 5 years of follow-up, 48% of patients treated with PDT who achieved complete ablation of high-grade dysplasia maintained the response, compared with 4% of those on proton pump inhibitor alone (P<0.0001) [33].
As with APC and multipolar electrocautery, PDT does not work in all patients. In a large retrospective case series of 363 patients who underwent treatment of dysplastic BE with PDT, 12.7% did not achieve eradication of their dysplasia. Of the 261 patients with a median 36 months of follow-up, 17.3% had recurrence of dysplasia during that time [34]. Smoking, residual BE after ablation, and increased age were associated with recurrence of dysplasia in this cohort [34].
Several side effects are associated with PDT, including chest pain, which is nearly ubiquitous, and stricture formation in as many as 36% of patients [10, 33]. Because of the use of a systemic agent, several side-effects are specific to PDT. Skin rash can occur when patients who have taken the photosensitizing agent are exposed to light. The skin rash is avoided by taking precautions to avoid light, but has been reported at rates as high as 69% of patients [10]. Permanent skin scarring may result after severe rash [10]. Mild elevation of liver tests has also been reported in 12% to 78% of patients receiving 5-aminolevulinic acid [35, 36].
Comparative Trials Including Multipolar Electrocautery, APC, and PDT
APC and multipolar electrocautery were compared to each other in two trials of patients with nondysplastic BE or low-grade dysplasia [27, 28]. In a study by Dulai et al. [27], no significant difference was demonstrated in ablation rates between groups: the proportion of patients achieving histologic ablation at the post-treatment surveillance endoscopy (4 to 8 weeks after the final therapy session) was 81% in the multipolar electrocautery group, compared with 65% in the APC group (P=0.21) [27]. The duration of follow-up was longer in the study reported by Sharma et al. [28] with a minimum 2-year follow-up period, but there was still no difference in sustained response between groups, as 75% achieved histologic ablation in the multipolar electrocautery group compared with 63.1% ablation in the APC group (P=0.49).
Results of studies that compared PDT with APC do not clearly demonstrate that either method of ablation is superior to the other. Thirteen patients with nondysplastic BE or BE with low-grade dysplasia randomly assigned to receive 5-aminolevulinic acid and a fractionated dose of PDT had a 33%rateofcomplete histologicresponseafter the initial dose, compared with a 36% response rate in the 14 patients who received APC [35]. A similar study of 68 patients with nondysplastic BE revealed a complete response to treatment in 50% of patients in the PDT arm after a median of two treatments, compared with a 97% response in the APC arm after a median of three treatments (P<0.0001) [36].
In these studies, subsquamous epithelium or buried glands continue to pose a common problem in the APC groups. Seven of nine patients who had BE epithelium detected after ablation with APC had buried glands detected, compared with one of the 26 patients in the PDT treatment group [35]. In a different study, buried glands were detected at similar rates (24% vs 21%) in the patients treated with PDT and APC, respectively [36].
Overall, although PDT shows effectiveness in ablating BE, the side effect profile with photosensitivity, elevated liver tests, and high stricture rates have caused it to fall from favor, especially as newer methods of ablation have been developed.
Radiofrequency Ablation
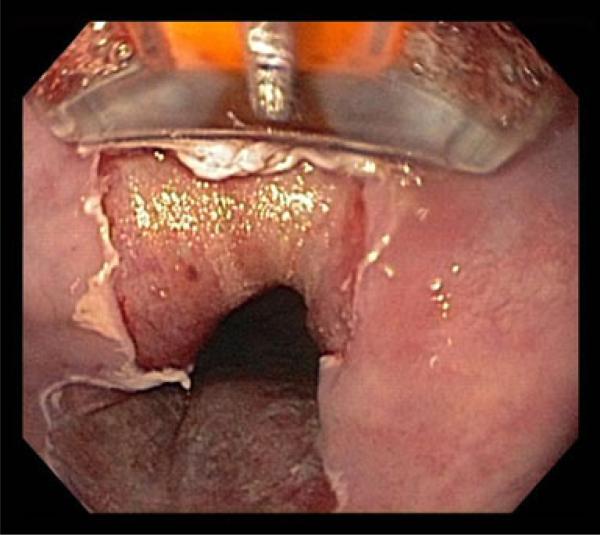
One of the newer treatments of BE is radiofrequency ablation. In this technology, regularly spaced positive and negative electrodes cover the surface of a device that is applied to the esophageal mucosa. Because the amount of energy and the duration of exposure of the energy are known, a reliable depth of injury is obtained. The devices now come in both a circumferential and focal form, allowing for treatment of both large and small amounts of diseased tissue (Fig. 1).
Fig. 1.
Focal radiofrequency ablation of Barrett's esophagus
Radiofrequency Ablation of Nondysplastic Tissue
In the Ablation of Intestinal Metaplasia (AIM) trial, balloon-based circumferential treatment of the esophagus with radiofrequency ablation was tested between 2003 and 2005 in 32 patients with nondysplastic BE using a dosimetry phase (AIM-I), followed by an effectiveness phase in 70 patients (AIM-II) [37]. Overall, in the effectiveness phase, BE was successfully ablated in 70% of patients and complication rates were very low, with fever in three patients, no strictures, and no evidence of buried glands [37].
Of the initial cohort of 70 patients enrolled in AIM-II, 62 participated in follow-up, and received additional focal radiofrequency ablation if needed [38]. After 30 months, 97% of patients had no histologic evidence of BE after an additional mean of 1.9 focal ablation procedures [38]. After 5 years of follow-up, 46 of 50 (92%) patients demonstrated complete response of BE and no buried glands were identified [39•].
These results demonstrate a good long-term response to radiofrequency ablation in nondysplastic BE, and a low side-effect profile. For complete eradication of BE, multiple treatments (usually a combination of circumferential and focal) are needed. However, the impact on cancer development or mortality in nondysplastic BE remains unclear. Such data will be difficult to generate experimentally, given the low rate of progression of nondysplastic BE.
Several small studies of radiofrequency ablation with sample sizes of 10 to 20 patients have been reported, including mixes of patients with nondysplastic BE, low-grade dysplasia, and high-grade dysplasia; generally, response rates were good, reaching 100% in some of the studies, but follow-up periods were short [40–44]. In these early reports, complication rates were very low.
Radiofrequency Ablation in Dysplastic BE
After the initial assessment of radiofrequency ablation in mostly nondysplastic Barrett's esophagus, this technology was applied to patients with dysplastic BE. In a prospective cohort study of 63 patients with BE with dysplasia, 79% of patients had complete ablation of BE and 89% had no evidence of dysplasia over a median follow-up of 23 months [45].
In a multicenter registry of high-grade dysplasia in 142 patients at 16 sites, after a median of one radiofrequency ablative treatment, and a median 12 months of follow-up, 54% of patients had no evidence of BE whereas 90% had no evidence of dysplasia [46]. One patient developed a stricture in this cohort. Two patients underwent esophagectomy for suspicious findings identified at the 3-month follow-up, and intramucosal carcinoma was found at esophagectomy in both patients [46]. It is important to note that this series was performed prior to the availability of the focal ablation device, which likely impacted the rate of complete eradication.
The first randomized, controlled trial of radiofrequency ablation was published in 2009 and included 127 patients with dysplastic BE (64 low-grade dysplasia and 63 high-grade dysplasia), who were randomly assigned to either radiofrequency ablation (84 patients) or sham-controlled procedure (43 patients) [9•]. In this trial, 77.4% of the ablation group achieved complete ablation of BE compared with 2.3% of the control group (P<0.001). In the low-grade dysplasia arm, dysplasia was eradicated in 90.5% of patients compared with 22.7% of those in the control arm (P<0.001). In the high-grade dysplasia arm, dysplasia was eradicated in 81.0% of patients compared to 19.0% of the control group (P<0.001). Overall, disease progression occurred in 3.6% of the treatment group, compared with 16.3% of the control group over 12 months of follow-up. Of patients with high-grade dysplasia, four patients (19.0%) developed cancer in the control arm compared with one patient in the ablation arm (2.4%) (P=0.04) [9•].
The randomized, controlled trial of radiofrequency ablation also demonstrated low complication rates, with infrequent reports of significant chest pain (3%) and a 6% stricture rate [9•].
In patients with high-grade dysplasia, any suspicious or nodular areas should be evaluated with endoscopic ultrasound and cross-sectional imaging as necessary. For lesions that do not require surgical intervention, endoscopic mucosal resection can be used to remove nodular areas, followed by radiofrequency ablation of remaining BE. Using this strategy, in a multicenter European cohort of 24 patients with high-grade dysplasia or low-grade intra-epithelial neoplasia, after endoscopic resection of the lesion followed by radiofrequency ablation, 88% of patients attained complete eradication of all BE [47]. After median follow-up of 22 months, one patient had recurrence of BE at the 6-month mark [47].
Radiofrequency Ablation in Long-Segment BE
In a single-center case series of 66 patients (18% had high-grade dysplasia), 13 of whom either had previously undergone or concurrently underwent fundoplication, short-term follow-up at 3 to 6 months after circumferential radiofrequency ablation revealed 59% of patients with evidence of complete eradication of BE by visual inspection [48]. This series included nine patients with long-segment BE greater than 10 cm. Four patients in this series developed strictures; three of the four strictures were in patients with greater than 12 cm of BE, prompting the author to conclude that no more than 6 cm of BE should be ablated at a time [48]. A series of 25 patients with ultra-long BE, defined as ≥8 cm, describes eradication of BE in 78.5% of patients and stricture formation in two patients (8%), which is higher than the stricture rate in most radiofrequency ablation studies, supporting the idea that stricture rates may be increased in those with long-segment BE [49].
Cryotherapy
An emerging therapy for Barrett's esophagus is cryotherapy, a treatment that uses endoscopically guided application of liquid nitrogen or liquid carbon dioxide in a spray. A prospective series of eleven patients with longstanding BE treated with cryotherapy was reported in 2005, and 78% of these patients had complete ablation of BE without evidence of BE at 6 months [50]. A retrospective case series of 31 patients from a single site during the development of this technique from 2005 to 2008 spanned three versions of the commercial device [51]. This study population was a relatively sick group of patients with either comorbidities making them poor candidates for esophagectomy or refusal of surgical intervention. Many of the patients had failed other endoscopic treatments for BE. Although complete response was low after one treatment at 17%, eventually, 90% of patients had some response and 30% to 40% of patients had a persistent response at 1 year [51]. This study demonstrated the safety of cryotherapy and noted that nearly all patients (97%) were able to participate in normal activities and eat normally the day after the procedure [51].
In a larger, multicenter, retrospective cohort study of 98 patients, many of whom had undergone previous therapy for BE, 60 had completed therapy at the time of publication and of these, 97% had eradication of high-grade dysplasia, and 57% had eradication of all metaplasia after a mean total of four treatments [57•]. Follow-up in this study was a mean of 10.5 months, and the long-term durability of the response to cryotherapy remains unknown. However, the safety profile of cryotherapy is excellent, and in the full cohort of 98 patients, 333 treatments were performed: 3% of patients developed strictures, 2% of patients had significant chest pain requiring narcotics, and one patient experienced disease progression despite therapy [57•].
Early data regarding cryotherapy in treatment of BE are promising. Longer term outcomes data are necessary, as are further data regarding side effect profile. Further complicating the interpretation of existing data is the fact that many of the patients in the existing studies had previously failed other ablation techniques.
Endoscopic Mucosal Resection
Endoscopic mucosal resection has been touted as an aggressive treatment of BE. Initially, endoscopic mucosal resection was used for focal areas of nodular dysplasia, or early cancer [52]. Focal endoscopic resection can be used in combination with other ablative strategies. For example, a nodule might be removed using endoscopic mucosal resection before the rest of the BE is ablated using radiofrequency ablation.
Recently, however, complete Barrett's eradication using primarily endoscopic mucosal resection has been reported as a treatment for BE. This technique may be called stepwise radical endoscopic mucosal resection, circumferential endoscopic mucosal resection, or complete Barrett's eradication-endoscopic mucosal resection (CBE-EMR); regardless of moniker, the goal is to endoscopically resect all mucosa containing BE. In an early case series of patients treated with radical endoscopic mucosal resection, recurrence of BE was limited to 1 of 32 patients, although it should be noted that APC was also used for additional ablation as needed [31]. In that series, the one recurrence took the form of subsquamous nondysplastic BE that was detected after 2 years during which the patient was considered BE-free [31]. In a recent abstract from this same group, the stricture rate after circumferential endoscopic mucosal resection was prohibitively high at 47% [53].
In a large, four-center, European retrospective cohort study of stepwise radical endoscopic resection for BE in 169 patients, 85% of patients achieved complete ablation of BE over a median follow-up of 32 months [54•]. APC was used to treat residual areas of BE in 61% of patients. One patient developed metastatic esophageal adenocarcinoma. As with the other studies of radical endoscopic mucosal resection, the major problem with this therapy is the very high rate of esophageal strictures requiring dilation in 50% of patients [54•].
Comparison of Radical Endoscopic Resection and Radiofrequency Ablation
A randomized, multicenter, European study compared stepwise radical endoscopic resection with radiofrequency ablation and presented in abstract form results from 43 patients with median follow-up of 13 months in 2009 [55]. Both types of treatment were performed with the goal of complete eradication of all intestinal metaplasia. In the radical endoscopic resection group, 96% of patients achieved eradication of all intestinal metaplasia. There was a comparable response rate of 95% in the radiofrequency ablation group. In the radical endoscopic resection group, an average of two therapeutic sessions was required to reach this endpoint, compared to three sessions in the radiofrequency ablation group [55]. Yet complication rates were quite different between the groups. The radical endoscopic resection group had bleeding in 23% of cases compared with 5% bleeding rate (delayed bleeding) in the radiofrequency ablation group. Stricture formation differed dramatically between groups, with an 86% stenosis rate in the radical endoscopic resection group compared with a 14% stenosis rate in the radiofrequency ablation group [55].
The extremely high stricture rate makes circumferential endoscopic mucosal resection a much less appealing treatment choice for the complete ablation of BE. Focal endoscopic resection can be used to treat local lesions, but strictures will be more common in these areas.
Safety of Barrett's Esophagus Ablation
In studies reporting outcomes for ablation of BE, complication rates vary substantially (Table 2). Some of this variation is from differences in the ablative techniques themselves, but some is explained by differences in the methods of recording and reporting complications and treatment-related symptoms.
Of the studies reviewed here, 15 described as a study outcome a measurement of complications and/or safety data. Seventeen studies described an assessment of side effects, complications, or symptoms in the methods. The methods used to measure and describe complications varied widely between studies. Thirteen studies described complications using a formal assessment of symptoms, and the most rigorous of these used standardized methods to measure symptoms such as patient-reported outcome indices and visual analog scales. In seven studies, providers called patients to assess post-procedure symptoms and in two additional studies, providers reported asking patients to call them to report any problems post-procedure. In one study, patients were observed for 24 h after their procedure.
Overall, deaths were rare in the studies reviewed here, occurring in one patient after PDT. Perforations were also rare, occurring most frequently in patients undergoing endoscopic mucosal resection [54•]. Perforation occurred after a stricture-related dilation in one patient who had received PDT [10, 13•]. In one patient, perforation occurred during development of the cryospray technique because of over-distension of the stomach; measures are now taken to decompress the stomach during the procedure [51].
Hospitalizations were quite rare. Causes for hospitalization included severe chest pain, bleeding, fever, and mucosal tears. Bleeding was reported in association with endoscopic mucosal resection [54•], but has been reported in patients undergoing radiofrequency ablation [47, 49] and after administration of cryospray [57•].
Reflecting the usual circumferential nature of the damage induced by ablative therapy, strictures were one of the most common complications. Esophageal strictures most commonly developed after endoscopic mucosal resection, but have been reported in association with all types of ablation strategies including multipolar electrocautery, APC, PDT, cryospray, and radiofrequency ablation. In most cases, the strictures reported were treated with dilation (balloon or Savary), although in one study, no dilation of the reported stenosis was required [46]. Some of the strictures associated with radical endoscopic mucosal resection required additional treatment beyond standard serial dilation, such as temporary stent placement or incision therapy [31, 54•].
Outcomes after Ablation
Collectively, ablation studies demonstrate the ability of these techniques to destroy areas of BE, even if dysplasia is present in the esophagus, fostering repair of the injured mucosa with a neosquamous epithelium. However, in some cases, areas of BE return to the esophagus. Retreatment is possible, and in some of the studies described here, “touch-up” treatments to the ablated esophagus were routinely performed.
The primary outcomes for most of these studies included efficacy of ablation of BE and safety measures. Some studies compared treatments, but very few evaluated long-term outcomes such as cancer development. None of the studies followed patients for longer than 5 years, and the vast majority followed patients for much shorter time periods.
None of these studies assessed long-term mortality data. Answering questions about mortality benefit in patients with BE will require large studies with longer term follow-up. Given the cost and difficulty of maintaining randomized controlled trials, registry and other observational data will likely be important in addressing this aspect of ablative therapy.
Reflux symptoms may persist after ablation, and generally require different interventions and treatment strategies beyond ablation of BE [56].
Quality of life is an important patient-centered outcome that improves after ablation of BE. On a customized, 10-item quality-of-life questionnaire administered to 127 patients, in those who achieved complete eradication of metaplasia, patients reported significantly decreased worry about risk of developing cancer and needing an esophagectomy as compared to the sham-control group. Patients who did not achieve complete eradication of metaplasia, but had eradication of dysplasia, also demonstrated improvements in quality of life [13•]. Further assessment of these important patient-centered outcomes is necessary.
Conclusions
Ablative therapy for subjects with BE is an exciting, quickly evolving area. Data convincingly demonstrate the ability of multiple modalities to induce reversion to neosquamous epithelium. Additionally, data substantiate a decreased risk of progression to cancer in subjects with dysplastic BE treated by two modalities, PDT and radiofrequency ablation. The long-term benefits of ablation require better definition, as do patient selection criteria for treatment.
Our lack of understanding of the pathogenesis of BE and esophageal adenocarcinoma also impacts our ability to interpret the significance of buried glands. If the deeper glands closer to the esophageal stroma are important in the pathogenesis of esophageal adenocarcinoma, then presence of these glands after ablation would be concerning. On the other hand, if communication with the noxious refluxate bathing the luminal surface is a requisite for degeneration to cancer, these buried glands may be less clinically relevant. Again, longer follow-up and additional translational research are needed.
Until we have more definitive data, one clinical approach is to offer radiofrequency ablation to patients with high-grade dysplasia after informing them of the risks and benefits of this and the competing management strategies. Nodular areas should be addressed with focal endoscopic mucosal resection prior to ablation, but wide-scale radical mucosal resection of all flat BE cannot currently be supported, given the high stricture rate associated with this approach. After the initial ablation, follow-up ablation with focal radiofrequency ablation can be performed. Until data tell us otherwise, surveillance after ablation should continue at regularly scheduled intervals with biopsies based on the highest degree of dysplasia prior to ablation.
With respect to low-grade dysplasia and nondysplastic disease, data are not available regarding decreased cancer incidence, and decisions to treat such patients invoke the surrogate marker of reversion to neosquamous epithelium as their rationale. In the case of low-grade dysplasia, certain conditions may merit special attention, including multifocal disease, disease confirmed by two expert pathologists, and disease in young people, who presumably have more life-years to develop advanced disease. Treatment of any subject with low-grade dysplasia should involve a frank discussion about what we know and do not know regarding ablative therapies. In nondysplastic disease, the risks of cancer development are even lower. Because these patients are earlier in their disease progression, continued endoscopic surveillance while more data accumulate to clarify the role of ablation in these subjects may be a reasonable strategy.
Footnotes
Disclosure Conflicts of interest: K.S. Garman—grants, American College of Gastroenterology Junior Faculty Development Award, and the Emeline Brown Cancer Genomics Research Fund; N.J. Shaheen—consultancies, CSA Medical, AstraZeneca, Takeda Pharmaceuticals, NeoGenomics, and OncoScope, and grants, BARRX Medical, AstraZeneca, NeoGenomics, Takeda Pharmaceuticals, CSA Medical, and OncoScope.
Contributor Information
Katherine S. Garman, Division of Gastroenterology, Institute for Genome Science and Policy, Duke University, Box 3913 DUMC, Durham, NC 27710, USA katherine.garman@duke.edu Durham VA Medical Center, 508 Fulton St., Durham, NC 27705, USA.
Nicholas J. Shaheen, Center for Esophageal Diseases and Swallowing, University of North Carolina School of Medicine, CB 7080, Chapel Hill, NC 27599–7080, USA nshaheen@med.unc.edu
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Eloubeidi MA, Mason AC, Desmond RA, El-Serag HB. Temporal trends (1973–1997) in survival of patients with esophageal adenocarcinoma in the United States: a glimmer of hope? Am J Gastroenterol. 2003;98:1627–33. doi: 10.1111/j.1572-0241.2003.07454.x. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Polednak AP. Trends in survival for both histologic types of esophageal cancer in US surveillance, epidemiology and end results areas. Int J Cancer. 2003;105:98–100. doi: 10.1002/ijc.11029. [DOI] [PubMed] [Google Scholar]
- 4.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184–7. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rex DK, Cummings OW, Shaw M, et al. Screening for Barrett's esophagus in colonoscopy patients with and without heartburn. Gastroenterology. 2003;125:1670–7. doi: 10.1053/j.gastro.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 6.Hayeck TJ, Kong CY, Spechler SJ, et al. The prevalence of Barrett's esophagus in the US: estimates from a simulation model confirmed by SEER data. Dis Esophagus. 2010;23:451–7. doi: 10.1111/j.1442-2050.2010.01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaheen NJ, Crosby MA, Bozymski EM, Sandler RS. Is there publication bias in the reporting of cancer risk in Barrett's esophagus? Gastroenterology. 2000;119:333–8. doi: 10.1053/gast.2000.9302. [DOI] [PubMed] [Google Scholar]
- 8.Sikkema M, de Jonge PJ, Steyerberg EW, Kuipers EJ. Risk of esophageal adenocarcinoma and mortality in patients with Barrett's esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2010;8:235–44. doi: 10.1016/j.cgh.2009.10.010. quiz e32. [DOI] [PubMed] [Google Scholar]
- 9 •.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med. 2009;360:2277–88. doi: 10.1056/NEJMoa0808145. [This randomized clinical trial evaluated radiofrequency ablation in patients with dysplastic BE.] [DOI] [PubMed] [Google Scholar]
- 10.Overholt BF, Lightdale CJ, Wang KK, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett's esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc. 2005;62:488–98. doi: 10.1016/j.gie.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 11.Konda VJ, Ross AS, Ferguson MK, et al. Is the risk of concomitant invasive esophageal cancer in high-grade dysplasia in Barrett's esophagus overestimated? Clin Gastroenterol Hepatol. 2008;6:159–64. doi: 10.1016/j.cgh.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Crockett SD, Lippmann QK, Dellon ES, Shaheen NJ. Health-related quality of life in patients with Barrett's esophagus: a systematic review. Clin Gastroenterol Hepatol. 2009;7:613–23. doi: 10.1016/j.cgh.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13 •.Shaheen NJ, Peery AF, Hawes RH, et al. Quality of life following radiofrequency ablation of dysplastic Barrett's esophagus. Endoscopy. 2010;42:790–9. doi: 10.1055/s-0030-1255780. [This article is one of the few reports of quality of life in patients with BE who undergo ablation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prasad GA, Wang KK, Buttar NS, et al. Long-term survival following endoscopic and surgical treatment of high-grade dysplasia in Barrett's esophagus. Gastroenterology. 2007;132:1226–33. doi: 10.1053/j.gastro.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inadomi JM, Somsouk M, Madanick RD, et al. A cost-utility analysis of ablative therapy for Barrett's esophagus. Gastroenterology. 2009;136:2101–14. e1–6. doi: 10.1053/j.gastro.2009.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curvers WL, ten Kate FJ, Krishnadath KK, et al. Low-grade dysplasia in Barrett's esophagus: overdiagnosed and underestimated. Am J Gastroenterol. 2010;105:1523–30. doi: 10.1038/ajg.2010.171. [DOI] [PubMed] [Google Scholar]
- 17.Montgomery E, Bronner MP, Goldblum JR, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol. 2001;32:368–78. doi: 10.1053/hupa.2001.23510. [DOI] [PubMed] [Google Scholar]
- 18.Ajumobi A, Bahjri K, Jackson C, Griffin R. Surveillance in Barrett's esophagus: an audit of practice. Dig Dis Sci. 2010;55:1615–21. doi: 10.1007/s10620-009-0917-y. [DOI] [PubMed] [Google Scholar]
- 19.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett's esophagus. Am J Gastroenterol. 2008;103:788–97. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 20.Lichtenstein DR, Cash BD, Davila R, et al. Role of endoscopy in the management of GERD. Gastrointest Endosc. 2007;66:219–24. doi: 10.1016/j.gie.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 21.Fleischer DE, Odze R, Overholt BF, et al. The case for endoscopic treatment of non-dysplastic and low-grade dysplastic Barrett's esophagus. Dig Dis Sci. 2010;55:1918–31. doi: 10.1007/s10620-010-1218-1. [DOI] [PubMed] [Google Scholar]
- 22.Falk GW. Radiofrequency ablation of Barrett's esophagus: let's not get ahead of ourselves. Dig Dis Sci. 2010;55:1811–4. doi: 10.1007/s10620-010-1292-4. [DOI] [PubMed] [Google Scholar]
- 23.Rees JR, Lao-Sirieix P, Wong A. Fitzgerald RC: Treatment for Barrett's oesophagus. Cochrane Database Syst Rev. 2010:CD004060. doi: 10.1002/14651858.CD004060.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Sampliner RE, Fennerty B, Garewal HS. Reversal of Barrett's esophagus with acid suppression and multipolar electrocoagulation: preliminary results. Gastrointest Endosc. 1996;44:532–5. doi: 10.1016/s0016-5107(96)70004-4. [DOI] [PubMed] [Google Scholar]
- 25.Sampliner RE, Faigel D, Fennerty MB, et al. Effective and safe endoscopic reversal of nondysplastic Barrett's esophagus with thermal electrocoagulation combined with high-dose acid inhibition: a multicenter study. Gastrointest Endosc. 2001;53:554–8. doi: 10.1067/mge.2001.114418. [DOI] [PubMed] [Google Scholar]
- 26.Sharma P, Bhattacharyya A, Garewal HS, Sampliner RE. Durability of new squamous epithelium after endoscopic reversal of Barrett's esophagus. Gastrointest Endosc. 1999;50:159–64. doi: 10.1016/s0016-5107(99)70218-x. [DOI] [PubMed] [Google Scholar]
- 27.Dulai GS, Jensen DM, Cortina G, et al. Randomized trial of argon plasma coagulation vs. multipolar electrocoagulation for ablation of Barrett's esophagus. Gastrointest Endosc. 2005;61:232–40. doi: 10.1016/s0016-5107(04)02576-3. [DOI] [PubMed] [Google Scholar]
- 28.Sharma P, Wani S, Weston AP, et al. A randomised controlled trial of ablation of Barrett's oesophagus with multipolar electro-coagulation versus argon plasma coagulation in combination with acid suppression: long term results. Gut. 2006;55:1233–9. doi: 10.1136/gut.2005.086777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bright T, Watson DI, Tam W, et al. Randomized trial of argon plasma coagulation versus endoscopic surveillance for barrett esophagus after antireflux surgery: late results. Ann Surg. 2007;246:1016–20. doi: 10.1097/SLA.0b013e318133fa85. [DOI] [PubMed] [Google Scholar]
- 30.Dulai GS, Guha S, Kahn KL, et al. Preoperative prevalence of Barrett's esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology. 2002;122:26–33. doi: 10.1053/gast.2002.30297. [DOI] [PubMed] [Google Scholar]
- 31.Chennat J, Konda VJ, Ross AS, et al. Complete Barrett's eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma–an American single-center experience. Am J Gastroenterol. 2009;104:2684–92. doi: 10.1038/ajg.2009.465. [DOI] [PubMed] [Google Scholar]
- 32.Ackroyd R, Brown NJ, Davis MF, et al. Photodynamic therapy for dysplastic Barrett's oesophagus: a prospective, double blind, randomised, placebo controlled trial. Gut. 2000;47:612–7. doi: 10.1136/gut.47.5.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Overholt BF, Wang KK, Burdick JS, et al. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett's high-grade dysplasia. Gastrointest Endosc. 2007;66:460–8. doi: 10.1016/j.gie.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 34.Badreddine RJ, Prasad GA, Wang KK, et al. Prevalence and predictors of recurrent neoplasia after ablation of Barrett's esophagus. Gastrointest Endosc. 2010;71:697–703. doi: 10.1016/j.gie.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hage M, Siersema PD, van Dekken H, et al. 5-aminolevulinic acid photodynamic therapy versus argon plasma coagulation for ablation of Barrett's oesophagus: a randomised trial. Gut. 2004;53:785–90. doi: 10.1136/gut.2003.028860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelty CJ, Ackroyd R, Brown NJ, et al. Endoscopic ablation of Barrett's oesophagus: a randomized-controlled trial of photodynamic therapy vs. argon plasma coagulation. Aliment Pharmacol Ther. 2004;20:1289–96. doi: 10.1111/j.1365-2036.2004.02277.x. [DOI] [PubMed] [Google Scholar]
- 37.Sharma VK, Wang KK, Overholt BF, et al. Balloon-based, circumferential, endoscopic radiofrequency ablation of Barrett's esophagus: 1-year follow-up of 100 patients. Gastrointest Endosc. 2007;65:185–95. doi: 10.1016/j.gie.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 38.Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic ablation of Barrett's esophagus: a multicenter study with 2.5-year follow-up. Gastrointest Endosc. 2008;68:867–76. doi: 10.1016/j.gie.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 39 •.Fleischer DE, Overholt BF, Sharma VK, et al. Endoscopic radiofrequency ablation for Barrett's esophagus: 5-year outcomes from a prospective multicenter trial. Endoscopy. 2010;42:781–9. doi: 10.1055/s-0030-1255779. [This study presents some of the longest follow-up data regarding ablation of nondysplastic BE as presented in the AIM-II study.] [DOI] [PubMed] [Google Scholar]
- 40.Roorda AK, Marcus SN, Triadafilopoulos G. Early experience with radiofrequency energy ablation therapy for Barrett's esophagus with and without dysplasia. Dis Esophagus. 2007;20:516–22. doi: 10.1111/j.1442-2050.2007.00728.x. [DOI] [PubMed] [Google Scholar]
- 41.Gondrie JJ, Pouw RE, Sondermeijer CM, et al. Stepwise circumferential and focal ablation of Barrett's esophagus with high-grade dysplasia: results of the first prospective series of 11 patients. Endoscopy. 2008;40:359–69. doi: 10.1055/s-2007-995567. [DOI] [PubMed] [Google Scholar]
- 42.Gondrie JJ, Pouw RE, Sondermeijer CM, et al. Effective treatment of early Barrett's neoplasia with stepwise circumferential and focal ablation using the HALO system. Endoscopy. 2008;40:370–9. doi: 10.1055/s-2007-995589. [DOI] [PubMed] [Google Scholar]
- 43.Sharma VK, Kim HJ, Das A, et al. A prospective pilot trial of ablation of Barrett's esophagus with low-grade dysplasia using stepwise circumferential and focal ablation (HALO system). Endoscopy. 2008;40:380–7. doi: 10.1055/s-2007-995587. [DOI] [PubMed] [Google Scholar]
- 44.Hernandez JC, Reicher S, Chung D, et al. Pilot series of radiofrequency ablation of Barrett's esophagus with or without neoplasia. Endoscopy. 2008;40:388–92. doi: 10.1055/s-2007-995747. [DOI] [PubMed] [Google Scholar]
- 45.Sharma VK, Jae Kim H, Das A, et al. Circumferential and focal ablation of Barrett's esophagus containing dysplasia. Am J Gastroenterol. 2009;104:310–7. doi: 10.1038/ajg.2008.142. [DOI] [PubMed] [Google Scholar]
- 46.Ganz RA, Overholt BF, Sharma VK, et al. Circumferential ablation of Barrett's esophagus that contains high-grade dysplasia: a U.S. Multicenter Registry. Gastrointest Endosc. 2008;68:35–40. doi: 10.1016/j.gie.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 47.Pouw RE, Wirths K, Eisendrath P, et al. Efficacy of radiofrequency ablation combined with endoscopic resection for barrett's esophagus with early neoplasia. Clin Gastroenterol Hepatol. 2010;8:23–9. doi: 10.1016/j.cgh.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Velanovich V. Endoscopic endoluminal radiofrequency ablation of Barrett's esophagus: initial results and lessons learned. Surg Endosc. 2009;23:2175–80. doi: 10.1007/s00464-009-0364-z. [DOI] [PubMed] [Google Scholar]
- 49.Vassiliou MC, von Renteln D, Wiener DC, et al. Treatment of ultralong-segment Barrett's using focal and balloon-based radio-frequency ablation. Surg Endosc. 2010;24:786–91. doi: 10.1007/s00464-009-0639-4. [DOI] [PubMed] [Google Scholar]
- 50.Johnston MH, Eastone JA, Horwhat JD, et al. Cryoablation of Barrett's esophagus: a pilot study. Gastrointest Endosc. 2005;62:842–8. doi: 10.1016/j.gie.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 51.Dumot JA, Vargo JJ, 2nd, Falk GW, et al. An open-label, prospective trial of cryospray ablation for Barrett's esophagus high-grade dysplasia and early esophageal cancer in high-risk patients. Gastrointest Endosc. 2009;70:635–44. doi: 10.1016/j.gie.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 52.Larghi A, Lightdale CJ, Memeo L, et al. EUS followed by EMR for staging of high-grade dysplasia and early cancer in Barrett's esophagus. Gastrointest Endosc. 2005;62:16–23. doi: 10.1016/s0016-5107(05)00319-6. [DOI] [PubMed] [Google Scholar]
- 53.Chennat J, Konda VJ, Hart J, et al. Complete Barrett's eradication endoscopic mucosal resection (CBE-EMR): long-term results of management of high grade dysplasia (HGD) and intramucosal carcinoma (IMC). Digestive Disease Week. 2010:S1517. 2010 May 2010. [Google Scholar]
- 54 •.Pouw RE, Seewald S, Gondrie JJ, et al. Stepwise radical endoscopic resection for eradication of Barrett's oesophagus with early neoplasia in a cohort of 169 patients. Gut. 2010;59:1169–77. doi: 10.1136/gut.2010.210229. [This is one of the largest studies of endoscopic mucosal resection as a strategy for ablation of BE.] [DOI] [PubMed] [Google Scholar]
- 55.Van Vilsteren FG, Pouw RE, Seewald S, et al. A multi-center randomized trial comparing stepwise radical endoscopic resection versus radiofrequency ablation for Barrett esophagus containing high-grade dysplasia and/or early cancer. Gastrointest Endosc. 2009;69:AB133–4. Abstract 939. [Google Scholar]
- 56.Eldaif SM, Lin E, Singh KA, et al. Radiofrequency ablation of Barrett's esophagus: short-term results. Ann Thorac Surg. 2009;87:405–10. doi: 10.1016/j.athoracsur.2008.11.043. discussion 10–1. [DOI] [PubMed] [Google Scholar]
- 57 •.Shaheen NJ, Greenwald BD, Peery AF, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett's esophagus with high-grade dysplasia. Gastrointest Endosc. 2010;71:680–5. doi: 10.1016/j.gie.2010.01.018. [This study represents one of the largest reports of cryotherapy for ablation of BE.] [DOI] [PMC free article] [PubMed] [Google Scholar]