Abstract
Since its release in 2006, the US Food and Drug Administration (FDA) final improved format for prescription drug labeling has revamped the comprehensiveness of drug inserts, including chemotherapy drugs. The chemotherapy drug “packets”, retrieved via the FDA website and other accredited drug information reporting agencies such as the Physician Drug Reference (PDR), are practically the only available unbiased summary of information. One objective is to impartially evaluate the reporting of useful pharmacokinetic parameters, in particular, Volume of Distribution (VD) and elimination half-life (t1/2), in randomly selected FDA approved chemotherapy drug inserts. The web-accessible portable document format (PDF) files for 30 randomly selected chemotherapy drugs are subjected to detailed search and the two parameters of interest are tabulated. The knowledge of the two parameters is essential in directing patient care as well as for clinical research and since the completeness of the core FDA recommendations has been found deficient, a detailed explanation of the impact of such deficiencies is provided.
Keywords: FDA drug inserts, Volume of Distribution (VD), elimination half-life (t1/2), database
Introduction
The development of chemotherapy drugs like the evolution in the formulation of any other consumer drug is an extremely expensive time consuming process and includes several experimental steps to establish its characteristics. These steps involve numerous in-vivo and in-vitro processes that will add to the list of relevant parameters that influences drugability of a given target [1]. When a particular chemotherapy drug is released for public consumption, all the acquired information is then compiled in a supposedly comprehensive patient package insert. These FDA approved drug inserts [2, 3] are intended to provide the patient with enough data to alert them to the benefit and risks of a particular drug. The inserts are also used by the clinician or physician to plan the chemotherapy regimens correctly, asses the drug’s efficacy and safety, and personalize it to the patient under treatment [4]. For example and as explained in the FDA website [2], poorly excreted drugs may be contraindicated in patients who have kidney problems; similarly poorly metabolized drugs may harm patients with liver problems. Finally the information amassed from these inserts is used extensively by researchers for the selection of their candidate drugs and in the planning of specific in-vivo, in-vitro [5], and in-silico tests to validate them [6].
Considering the above statements it is evident that the lack of proper documentation of important pharmacokinetic parameters will not only directly affect patient care, but it will also affect proper care indirectly by negatively influencing development of new drugs. Our hypothesis based on previous work [7–9], is that there unfortunately exist a significant deficit in the reporting of various parameters in the chemotherapy drug inserts and an obvious outcome will hopefully be a push towards reinforcing strict criteria for constructing the packets.
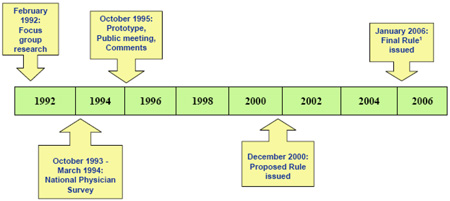
The FDA’s mission is to make sure that all approved products specifically medicines such as chemotherapy drugs sold in the United States are safe, effective, and pure. To insure the proper standardization and accuracy of all publicly released information on drug products, a package insert (also known as prescription drug product insert or professional labeling) is compiled and distributed by the drug manufacturer, after FDA review and approval. In 2006, the standard format for the package insert was changed in an attempt to make it more user-friendly and is an efficient resource tool for patients, practitioners, and researchers [3]. It was expected that the new package insert format will enhance rapid access to important pharmacologic information, improve patient safety, and could contribute to the process for the development of new improved drugs. This initiative was the result of a focused approach over a 14 year span as shown in the following illustration adopted from the FDA health professionals training course [3].
A proper review of the implementation steps and final result of the FDA 2006 improved format for prescription drug labeling is performed, in order to correlate the findings of the expected deficiencies in the sampled inserts. While the FDA addressed most of the deficiencies in the old format, their recommendations are lacking in clinical pharmacology specifics [10, 11]. Their revision of the safety requirements is elaborate; however their suggestion on including all the important pharmacokinetics parameters is not as strong [12]. Important parameters such as: Oncogenicity, Teratogenicity, Mutagenicity, Human Intestinal Absorption, Plasma Protein Binding, Water Solubility, Bioavailability, Elimination Half-Life, Rate of Absorption, Blood Brain Barrier, Neurotoxicity, pKa, Volume of Distribution, and log P, were not addressed equally. While few were re-emphasized and re-categorized such as oncogenicity, and teratogenicity, the more specific pharmacokinetic parameters like elimination half-life, and Volume of Distribution, were not mentioned.
The objectives of the proposed research can be summarized as: first, to objectively retrieve the drug packets of the randomly selected chemotherapy drugs using secure FDA accredited drug reporting websites. While the FDA website is still the gold standard to such information, we also elected to use the “Physician Desk Reference” (PDR) website [13] as well. This way we insure wider coverage and a more comprehensive approach. Second, scrutinize these printed packets for the target parameters (viz. elimination half-life, and Volume of Distribution) to eventually create an easily searchable table to include all the acquired data. Third, to show that most of the randomly selected chemotherapy drugs information packets are deficient in reporting the above mentioned pharmacokinetics parameters. Fourth, to point out the need for a more comprehensive drug insert that is in complete compliance with the FDA recommendations since these items have a direct impact on the physicians’ decision, in the development of new drugs, and ultimately, in patient care.
Materials and Methods
The primary task involved randomly selecting 30 chemotherapy drugs using the FDA website and scrolling through the posted data base. An impartial randomizing method is used to select from the pull down list. The selected drugs are transcribed onto an MS (Microsoft) Excel spread-sheet in preparation of the final table formation. They are written in an alphabetical order and are assigned numbers from one to thirty. The inserts analyzed are for the following drugs: Abraxane® Afinito® Agrylin® Alimta® Arimidex® Aromasin® Arranon® Carac® Casodex® Clolar® Cosmegen® Cytoxan® Dacogen® Depocyt® Doxil® Eligard® Eloxatin® Evista® Faslodex® Fludara® Gemzar® Gleevec® Gliadel® Wafer Hexalen® Idamycin® Ixempra® Panretin® Paraplatin® Plenaxis® Targretin®
The MS Excel spread-sheet (shown below in Table 1.) is designed in a way to accept the following parameters: drug brand name, drug scientific name, intended use, and presence or absence of the targeted pharmacokinetics parameters (Volume of Distribution, elimination half-life,). The inserts retrieved online are in PDF format thus they are first converted to hard copies (printout) for easy searching ability. Each packet is assigned a number in accordance with its number in the MS Excel sheet. The data collection is done by reading each packet in detail and highlighting all the information needed. The two parameters of interest are searched for with special attention given to the way they are reported (if any).
Table 1.
FDA chemotherapy drug targeted pharmacokinetics database. Includes alphabetically sorted sampled drugs with related scientific names, classification, intended use, Volume of Distribution (l/m2) and elimination half-life (hourly value). NR (not reported), MPD (Myeloproliferative Disorder), CML (Chronic Myelocytic Leukemia), ER (Estrogen Receptor), ALL (Acute Lymphocytic Leukemia), AML (Acute Myelocytic Leukemia), WT (Wilms Tumor), ES (Euwings Sarcoma), MM (Multiple Myeloma), HL (Hodgkins Lymphoma), CLL (Chronic Lymphocytic Leukemia), MF (Mycosis Fungoides), MDS (Myelodysplastic Syndrome), KS (Kaposi Sarcoma), NSCLC (Non Small Cell Lung Cancer), Ph (Philadelphia), GIST (Gastrointestinal Stromal Tumor),
| Number | Drug brand name |
Drug scientific name |
Classification | Intended use | Volume of Distribution |
elimination half-life |
|---|---|---|---|---|---|---|
| 1 | AbraxaneCB | Paclitaxel | Inhibits cell division | Metastatic breast cancer | 632 | 27 |
| 2 | AfinitorCB | Everolimus | Kinase inhibitor, inhibits mTOR | Advanced Renal Cell Carcinoma | NR | NR |
| 3 | AgrylinCB | Anagrelide HCl | Platelet reducing agent | MPD, Polycythemia, CML | NR | 1.3 |
| 4 | AlimtaCB | Pemetrexed | Disrupt folate dependent metabolism | Mesothelioma, NSCLC | 16.1 | 3.5 |
| 5 | Arimidex | Anastrazole | aromataze inhibitor | Receptor+ breast cancer | NR | 50 |
| 6 | AromasinCB | Exemestane | aromataze inhibitor | ER+ breast cancer | NR | 24 |
| 7 | Arranon | Melarabine | Inhibit DNA synthesis | T-cell lymphoblastic leukemia/lymphoma | 197 | 0.5 |
| 8 | CaracCB | Flurouracil cream | Inhibit DNA synthesis | Actinic keratosis | NR | NR |
| 9 | Casodex | Bicalutamide | nonsteroidal antiandrogen | Prostatic cancer | NR | 5.8 |
| 10 | Clolar | Clofarabine | Inhibit DNA synthesis | ALL and AML | 1724 | 5.2 |
| 11 | CosmegenCB | Dactinomycin | Inhibit RNA synthesis | WT, Rhabdomyosarcoma, ES | NR | 36 |
| 12 | Cytoxan | Cyclophosphamide | Crosslinkage of DNA | Lymphoma, MM, HL, CLL, AML, MF | NR | 7.5 |
| 13 | DacogenCB | Decitabine | Inhibit DNA methyltransferase | MDS | NR | 0.51 |
| 14 | DepocytCB | Cytarabine | Inhibit DNA polymerase | Lymphoma, intrathecal | NR | 44.15 |
| 15 | DoxilCB | Doxorubicin Hcl | anthracycline topoisomerase inhibitor | Ovarian cancer, KS, MM | 700 | 4.7 |
| 16 | EligardCB | Leuprolide acetate | LH/RH anatgonist | Prostatic cancer | 27 | 3 |
| 17 | EloxatinCB | Oxaliplatin | platinum based agent | colorectal cancer | 440 | 196 |
| 18 | EvistaCB | Raloxifene | selective ER modulator | reduce invasive cancer | 2348 | 27.7 |
| 19 | Faslodex | Fulvestrant | Estrogen receptor antagonist | ER+ breast cancer | 4 | 960 |
| 20 | Fludara | Fludarabine | Inhibit DNA synthesis | Adult B-cell CLL | NR | 20 |
| 21 | GemzarCB | Gemcitabine | Inhibit DNA synthesis | Ovarian cancer, Breast cancer, NSCLC | 370 | 7.4 |
| 22 | GleevecCB | Imatinib Mesylate | Kinase inhibitor | Ph Chromosome+ CML, GIST | NA | 18 |
| 23 | GliadelCB Wafer | Camustine implant | Alkylating agent for DNA and RNA | High grade malignant Glioma | 3.25 | 0.36 |
| 24 | Hexalen | Altretamine | Synthetic cytotoxic agent | Ovarian cancer | NR | 7.5 |
| 25 | IdamycinCB | Idarubicin Hcl | Nucleic acid synthesis inhibitor via Topoisomerase II | AML in adults (M1-M7) | NR | 22 |
| 26 | IxempraCB | Ixabepilone | Microtubule inhibitor | Breast cancer | 1000 | 52 |
| 27 | PanretinCB | Alitretinoin 0.1% | Retinoic acid receptor antagonist | Kaposi Sarcoma in AIDS patients | NR | NR |
| 28 | ParaplatinCB | Carboplatin | Inhibit DNA synthesis | Advanced ovarian cancer | 16 | 4.25 |
| 29 | PlenaxisCB | Abarelix | GnRh Inhibitor | Advanced prostate cancer | 4040 | 316 |
| 30 | TargretinCB | Bexarotene | Retinoic acid receptor antagonist | Cutaneous t-cell lymphoma | NR | 7 |
CB (contains a Carbonyl group in its chemical structure).
The next step involved transcribing the information onto the MS Excel spread-sheet. The way of reporting the parameters is standardized so as to make it acceptable for comparison. In other words, in the reporting of a parameter (for example elimination half-life), in order to be accepted as a part of the information packet, it should provide meaningful information that can be compared to other drugs. Since such documentation is sometimes in the form of specific values as numbers or percentages, while in other instances it is just reported as a description (high, low, or medium), a standardized method is devised for each parameter to facilitate reporting. This is performed concomitantly with the transcription of the information into the Excel sheet. The final product is a detailed searchable table that made it easy to compare the reporting of the pharmacokinetics in question. The results are then analyzed and an objective measure of the observed deficiencies is deduced.
Results
The expected outcome based on this pilot study, is revealed in the major and significant deficiencies in the released inserts of the selected chemotherapy drugs (Table 1). We are able to show that the parameters of interest, particularly Volume of Distribution (VD), are missing in a substantial percentage of the packets tested (Table 2). The Volume of Distribution (VD) is a useful pharmacokinetic parameter that relates the amount of drug to its observed concentration. Even though it has no true physiological significance, its numerical value is indicative of the extent of distribution of the drug (since VD is used to estimate the amount of drug in the body, peak serum levels, and clearance). The elimination half-life (t1/2) is calculated from the serum level decay curve and is defined as the time necessary for the drug to be reduced to half of its pharmacologic, physiologic, or radiologic level in the body through various bodily processes. Elimination half-life is a dependent variable, related directly to the Volume of Distribution and inversely to clearance [14]. Hence, knowing both parameters is essential to calculate the proper dosage regimen for a patient. Furthermore, due to the increasing complexity and challenges of cancer chemotherapy makes it mandatory that the pharmacists be familiar with the complicated regimens and highly toxic agents used [15].
Table 2.
Reported % and Unreported % of the targeted pharmacokinetic parameters showing a large discrepancy between the reporting of the two parameters.
| Pharmacokinetic Parameter |
Reported (%) | Unreported (%) |
|---|---|---|
| Volume of Distribution | 46.6 | 53.4 |
| Elimination half-life | 90 | 10 |
| Total | 68.3 | 31.7 |
In anticipation for a collaborative study with a concurrent research project targeting Structure Activity Relationships (SARs), we also assessed those compounds (amongst the 30 chemotherapy drugs listed in Table 1) that contained at least one carbonyl group (C=O) within the drugs 3-D chemical structure. The Reported %, and Unreported %, of the target pharmacokinetic parameters (viz. Volume of Distribution and elimination half-life) was then correlated for the carbonyl containing compounds within the Excel data sheet (Table 3). As expected, there is no significant statistical difference for the data shown in Tables 2, and 3; between the percentages observed for the reported and unreported VD and t1/2 parameters.
Table 3.
Reported % and Unreported % of the targeted pharmacokinetic parameters in the 22 sampled chemotherapy drugs containing a carbonyl group.
| Pharmacokinetic Parameter in carbonyl group containing drugs |
Reported (%) | Unreported (%) |
|---|---|---|
| Volume of Distribution | 50 | 50 |
| Elimination half-life | 86.4 | 13.6 |
| Total | 68.2 | 31.8 |
Discussion
Clearly, the results show that there is marked deficiency in reporting of the Volume of Distribution parameter in the sampled inserts. While the reporting of the elimination half-life parameter looks sufficient (90%, as reported in Table 2.), it is less than optimal, as this major clinical parameter will have direct implication on management decisions ultimately resulting in suboptimal patient care. The new FDA recommendations for labeling [3] do not seem stringent [16] and there are no uniform manufacturer regulations. The results from this study now make it increasingly clear that the drug manufacturers are not abiding by these lenient rules. Additionally, in a recent novel project [7–9] related to the development and structure activity relationships (SARs) study of a commercially available searchable database [17] of unrelated structurally diverse consumer drugs, numerous useful pharmaceutical and pharmacological profiles were found to be missing from many of the individual common consumer drug package inserts. In order to guarantee drug safety and to maintain the integrity and reliability of experimental data, it is imperative that the parameters governing the events leading to pharmacological action when available should be mentioned in each packet [18].
The overwhelming cost of driving a drug from the discovery stage to its final consumer level cost a little over 800 million dollars and almost 12 years [19, 20]. Any modification to the system that can improve on these numbers will definitely help and the ultimate benefit will be in the reduction of the cost of the final product, thus making it more affordable. Lack of documentation of useful pharmacokinetic parameters in the drug inserts will impact the many approaches by which new chemotherapy drugs are formulated since drug companies usually rely on previous successful models [21]. In other words since the behavior of a specific drug in-vivo depends on its pharmacokinetics, the development of more efficient related chemotherapy drugs can be shortened if all of the targeted parameters are known. These parameters collectively referred to as ADME/Tox drug properties (absorption, distribution, metabolism, elimination and toxicity), are at the center of the success or failure of any drug [22] causing 50% to fail at the experimental levels, and up to 40% to be dismissed before that because of safety problems. Recently much effort has been put into in-silico prediction’s ability to accurately predict human absorption based on in-vitro data in an attempt to decrease time needed to select good candidate drugs [23]. Many other qualitative or semi-quantitative methods have also been developed to test these drugs and clear them for experimental stage [24]. These tests target specific pharmacokinetics such as intestinal absorption, drug metabolism, drug-drug interaction, as well as the two parameters screened for in this research. The importance of these parameters is also evident by the development of new in-silico screening tools [25] by the pharmaceutical industry to obtain the most accurate results as a means to cut down on the cost of developing new drugs. Management of all this biomolecular information requires efficient data storage and retrieval from integrated databases [26]. However, the success of such in-silico approaches to model, predict, and explain biological function depends primarily on the available data from previous experiments for the development of a core database that will serve as the source for organizing, storing, accessing, and visualizing—the various kinds of experimental data in concert with the diverse classes of computational models that attempt to capture their salient features [27].
In the same token, the importance of knowing the pharmacokinetic parameters is as important clinically as it is pharmacologically [28]. As chemotherapy drug recipients are usually sicker patients with multiple organ problems, the type and dosage of the chemotherapy drug is personalized to each patient. If a patient in need of a particular chemotherapy has kidney problems, special care should be taken about the “terminal elimination half life” [29]. While the risk of a poorly eliminated drug will outweigh its benefit, the dosage can be adjusted so as this risk is eliminated [14]. Similarly other parameters are of equal or more importance in directing patient care. We can thus imagine the disadvantages of the failure to mention these parameters in the drug inserts for modern in-silico prediction methods can now be of use in replacing missing data or extrapolating parameters into similar drugs [22, 30]. However, these methods have never been tested as a clinical tool to estimate missing data in a clinical setting and its application to treatment regimens. The chemical properties of drugs in general and chemotherapy drugs in particular, dictate their biological properties. In other words, variation in biological response can be expressed as a function of the variation in the chemical structure space [31]. Subsequently when a drug is in the stage of development, its characteristics can be tested in in-vitro experiments and later on by in-vivo methods. Hence, any acquired data should be reported in detail in the inserts published explaining the use of the drug to guide regulatory policy and to influence medical practice [18].
It is the belief of these authors that studies similar to the current endeavor will contribute fundamentally to scientific understanding of using core knowledge of pharmacokinetics to inform safe and effective prescribing [32]. They will also expose an issue resulting from lack of comprehensive reporting of pharmacokinetic parameters in chemotherapy drug inserts that could have significant implications for modern chemotherapy applications and research. The clinical impact of such projects is improving drug safety understanding by the end-user and is also paralleled by the added value to the drug developing researchers through elucidating targets and/or associated pathways, thus cutting new drug research related cost and time consumption [33]. The success of this and similar projects could open the door towards more elaborate studies dealing with general consumer drug packets and urge federal institutions to demand more comprehensive reporting strategies.
Acknowledgements
This research was supported by grant number 2 P2O RR016472-09 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). This IDeA Network of Biomedical Research Excellence (INBRE) grant to the state of Delaware was obtained under the leadership of the University of Delaware, and the authors sincerely appreciate their efforts.
Biographies

Malcolm J. D’Souza, Ph.D. (http://www.pharmainfo.net/dr-malcolm-j-dsouza)
Dr. Malcolm J. D’Souza is Professor of Chemistry at Wesley College, in Dover, Delaware. He has published over 50 peer-reviewed journal articles, over 150 conference proceedings, and has established a nationally recognized Wesley College Undergraduate Directed Research Program in Chemistry. There are 31 undergraduate co-authors on his list of peer-reviewed publications, and 10 Wesley College undergraduates from his laboratory have earned national awards. In 2009, he was nominated and identified by a selection committee for the 2009 Northern Illinois University (NIU) Golden Anniversary Alumni Award. For this award, he was selected as one of fifty distinguished alumni spanning the 50-year history of the college who have distinguished themselves either in their professional fields or through their involvement in civic, cultural, or charitable service and will now take a distinctive place among its nearly 70,000 alumni. In 2008, the Delaware American Chemical Society (DE-ACS) nominated Dr. D’Souza to the 2008 E. Emmett Reid Award for Excellence in Teaching at a Small College. Dr. D’Souza has also received 3 other teaching awards, a faculty research award, and currently serves as Guest Editor for a peer-reviewed journal. His current research efforts in bio-organic chemistry are supported by grant number 2 P2O RR016472-09 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). This IDeA Network of Biomedical Research Excellence (INBRE) grant to the state of Delaware was obtained under the leadership of the University of Delaware. Dr. D’Souza now serves as Co-Director for Undergraduate Research on this $17.4 million grant to Delaware.

Ghada J. Alabed (http://www.pharmainfo.net/ggergeos-0)
Ghada J. Alabed (B.S. Biology, May 2010) is a senior at Wesley College with a cumulative GPA of 3.87. She has earned several scholarships and honors, and is an active participant in the Wesley College Undergraduate Directed Research Program in Chemistry. Ghada recently lead a group of Wesley Science majors to organize fun activities in a Kids & Chemistry program for around four hundred K-12 kids at the Independence School, in Newark, Delaware. This event was organized by the DE-Section of the American Chemical Society (ACS). Ghada is scheduled to present the outcomes of this activity at the 239th National ACS Meeting in San Francisco, CA, March 21–25, 2010.
Contributor Information
Malcolm J. D’Souza, Email: dsouzama@wesley.edu.
Ghada J. Alabed, Email: Ghada.alabed@email.wesley.edu.
References and Notes
Ghada J. Alabed a Wesley College Biology major completed this project as part of the Senior Research Capstone Experience in the Department of Science. She received an INBRE supported Undergraduate Research Assistantship in the Directed Research Program in Chemistry at Wesley.
- 1.Kraljevic S, Stambrook PJ, Pavelic K. Accelerating Drug Discovery. EMBO Reports. 2004;5(9):837–842. doi: 10.1038/sj.embor.7400236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Food and Drug Administration (FDA) profiles. Retrieved (August–December, 2009) from http://www.fda.gov/cder/drug/default.htm.
- 3. Retrieved (August–December, 2009) from the FDA Training & Continuing Courses link http://www.fda.gov/Training/ForHealthProfessionals/ucm090590.htm.
- 4. Retrieved (December 3, 2009) from www.clinicaltrials.gov a service of the US National Institutes of Health.
- 5.Kumaresan C. In-Vitro In-Vivo Studies in Pharmaceutical Development. Pharmaceutical Reviews. 2008;6(2) ISSN 1918–5561. [Google Scholar]
- 6.Rajman I. PK/PD Modeling and Simulations: Utility in Drug Development. Drug Discovery Today. 2008;13(7–8):341–346. doi: 10.1016/j.drudis.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 7.D’Souza MJ, Koyoshi F. Extracting Relevant Information From DFA Drug Files to Create a Structurally Diverse Drug Database Using KnowItAll®. Pharmaceutical Reviews. 2008;7(3) ISSN 1918–5561. [PMC free article] [PubMed] [Google Scholar]
- 8.D’Souza MJ, Koyoshi F, Everett LM. Structure Activity Relationship (SAR) Patterns Observed Within a Series of Unrelated Common Consumer Drugs. Bioinformatics, Computational Biology, Genomics, and Chemoinformatics. 2009:1–6. ISBN: 978-1-60651-009-4. [Google Scholar]
- 9.D’Souza MJ, Koyoshi F, Everett LM. Structure Activity Relationships (SARs) Using a Structurally Diverse Drug Database: Validating Success of Predictor Tools. Pharmaceutical Reviews. 2009;7(5) ISSN 1918–5561. [PMC free article] [PubMed] [Google Scholar]
- 10.NSAID Package Insert Labeling Template. Retrieved (November–December, 2009) from http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm106230.pdf.
- 11.Mitka M. Drug Package Inserts get Mixed Reaction. JAMA. 2006;295:1110–1111. doi: 10.1001/jama.295.10.1110. [DOI] [PubMed] [Google Scholar]
- 12.Watson KT, Barash PG. The New Food and Drug Administration Drug Package Insert: Implications for Patient Safety and Clinical Care. Anesthesia Analgesia. 2009;108:211–218. doi: 10.1213/ane.0b013e31818c1b27. [DOI] [PubMed] [Google Scholar]
- 13.Physician Desk Reference (PDR) Retrieved (August–December, 2009) from the PDR website http://www.pdr.net/login/Login.aspx.
- 14.Greenblatt DJ. Elimination Half-Life of Drugs: Value and Limitations. Annual Review of Medicine. 1985;36:421–427. doi: 10.1146/annurev.me.36.020185.002225. [DOI] [PubMed] [Google Scholar]
- 15.Solimando DA, Jr, Waddell JA, Watts AJ. Docetaxel and Estramustine for Prostate Cancer. Hospital Pharmacy. 2009;44(6):473–479. [Google Scholar]
- 16.Avorn J. FDA Standards — Good Enough for Government Work? The New England Journal of Medicine. 2005;353(10):969–972. doi: 10.1056/NEJMp058174. [DOI] [PubMed] [Google Scholar]
- 17.D’Souza MJ. HaveItAll - ADME/Tox Experimental Databases Datasheet. Bio-Rad Laboratories; 2008. FDA Consumer Drug database – 2007. Bulletin # INF-96199. [Google Scholar]
- 18.Bradford GE, Elben CC. The Drug Package Insert and the PDR as Establishing the Standard of Care in Prescription Drug Liability Cases. Journal of the Missouri Bar. 2001;57(5) ISSN 0026-6485. [Google Scholar]
- 19.DiMasi JA, Hansen RW, Grabowski HG. The Price of Innovation: New Estimates of Drug Development Costs. Journal of Health Economics. 2003;22(2):151–185. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 20.Adams CP, Branter VV. Estimating The Cost Of New Drug Development: Is It Really $802 Million? Health Affairs. 2006;25(2):420–428. doi: 10.1377/hlthaff.25.2.420. [DOI] [PubMed] [Google Scholar]
- 21.Balant LP, Gex-Fabry M. Modelling During Drug Development. European Journal of Pharmaceutics and Biopharmaceutics. 2000;50(1):13–26. doi: 10.1016/s0939-6411(00)00083-7. [DOI] [PubMed] [Google Scholar]
- 22.Albert PL. Screening for Human ADME/Tox Drug Properties in Drug Discovery. 2007;6(7):357–366. doi: 10.1016/s1359-6446(01)01712-3. [DOI] [PubMed] [Google Scholar]
- 23.Jónsdóttir SÓ, Jørgensen FS, Brunak S. Prediction Methods and Databases Within Chemoinformatics: Emphasis on Drugs and Drug Candidates. Bioinformatics. 2005;21(10):2145–2160. doi: 10.1093/bioinformatics/bti314. [DOI] [PubMed] [Google Scholar]
- 24.Ekins S, Mestres J, Testa B. In Silico Pharmacology for Drug Discovery: Methods for Virtual Ligand Screening and Profiling. British Journal of Pharmacology. 2007;152(1):9–20. doi: 10.1038/sj.bjp.0707305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ekins S, Rose J. Meeting Report In Silico ADME/Tox: The State of The Art. Journal of Molecular Graphics and Modelling. 2002;20(4):305–309. doi: 10.1016/s1093-3263(01)00127-9. [DOI] [PubMed] [Google Scholar]
- 26.van Beek JHGM. Channeling the Data Flood: Handling Large-Scale Biomolecular Measurements In Silico. Proceedings of the IEEE. 2006;94(4):692–709. [Google Scholar]
- 27.National Institute of General Medical Sciences Quantitative and Systems Pharmacology Workshop. Retrieved (January 1, 2010) from http://www.nigms.nih.gov/News/Reports/PharmacologyConference20080925.htm.
- 28.Kastrissios H, Blaschke TF. Medication Compliance as a Feature in Drug Development. Annual Review of Pharmacology and Toxicology. 1997;37:451–475. doi: 10.1146/annurev.pharmtox.37.1.451. [DOI] [PubMed] [Google Scholar]
- 29.Gabardi S, Abramson S. Drug Dosing in Chronic Kidney Disease. Medical Clinics of North America. 2005;89(3):649–687. doi: 10.1016/j.mcna.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Tetko IV, Bruneau P, Mewes HW, Rohrer DC, Poda GI. Can We Estimate the Accuracy of ADME-Tox Predictions? Drug Discovery Today. 2006;15–16:700–707. doi: 10.1016/j.drudis.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Prabhakar YS, Gupta MK. Chemical Structure Indices in In-Silico Molecular Design. Scientia Pharmaceutica. 2008;76(2):101–132. [Google Scholar]
- 32.Maxwell S, Walley T. Teaching Safe and Effective Prescribing in UK Medical Schools: A Core Curriculum for Tomorrow's Doctors. British Journal of Clinical Pharmacology. 2003;55(6):496–503. doi: 10.1046/j.1365-2125.2003.01878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watson C, Woodcock J. Janet Woodcock Discusses the FDA and the Drug Development Process. Drug Discovery Today. 2004;9(13):548–550. doi: 10.1016/S1359-6446(04)03160-5. [DOI] [PubMed] [Google Scholar]