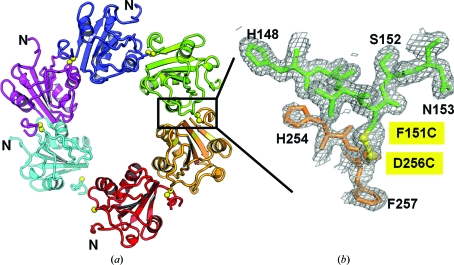
Figure 6.
(a) A ribbon diagram of the T-ag OBD triple-mutant hexamer structure looking down the sixfold axis. The different protomers are colored differently. The disulfide linkage is indicated by yellow spheres. The N-termini are labelled. A box is drawn around the region used in the close-up view. (b) A close-up of the 2F o − F c σA-weighted electron-density map contoured at 0.8σ around the protein–protein interface. Again, the different protomers are colored differently and the residues are shown as sticks and labeled. The disulfide bond is shown in yellow.