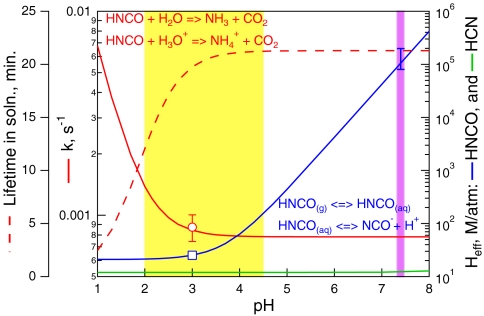
Fig. 4.
The Henry’s Law constant (blue) from our measurement at pH = 3 and the weak acid equilibrium relationship Eq. 4, first-order loss rate due to hydrolysis from the rate constants measured by Jensen (39) (solid red), and corresponding aqueous phase lifetime (dashed red) of HNCO vs. pH. The open red circle is our measurement of the first-order loss rate at pH = 3 (± 1σ) and the open blue square is our measurement of Heff at pH = 3. Also shown is the Henry’s Law constant for HCN (green). The yellow band indicates the range of pHs most characteristic of ambient aerosol, and the pink band indicates physiological pH, and the error bar at pH = 7.4, is the estimated uncertainty based on the uncertainties in Heff measured at pH = 3, (± 3 M/atm) and, pKa (± 0.2 pH units).