Figure 1.
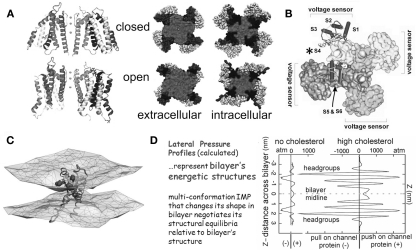
The structural basis of mechanosensitivity in VGCs. (A) Left, a Kv channel (Long et al., 2007) modeled in closed-like and open-like conformations and right (for intracellular and extracellular faces of the Kv) space-filling models of these states (modified from Chanda et al., 2005) and based on (Long et al., 2007) and refs therein). Due to its cruciform shape, the VGC’s lateral interactions with bilayer lipids are considerably more extensive than if the protein were cylindrical (the typical depiction before high resolution structures were available). Because of the substantial shape change of the closed-open transition, some of the free energy of the transition must be attributed to restructuring the lateral protein-lipid interface. (B) The 4 voltage sensors, whose gating charge is primarily in transmembrane segment S4 (asterisk), occupy the periphery (see Long et al., 2007). (C) A single activated-state voltage sensor domain modeled in lipid bilayer (the two water/lipid interfaces are depicted) showing that in its activated-state, the sensor locally deforms/thins the bilayer (modified from Krepkiy et al., 2009). (D) Bilayers, too have different structures (and hence energetics) that are dramatic and lipid-dependent, as seen from these lateral pressure profiles calculated for two simple bilayers, one without, one with cholesterol. Cholesterol thickens a bilayer (see Z axis) and increases its packing order. For any shape-changing integral membrane protein (IMP), the lateral pressure profile is an important component of the energetic landscape (for further explanation see Morris and Juranka, 2007b; Finol-Urdaneta et al., 2010; Morris, 2011a, b).