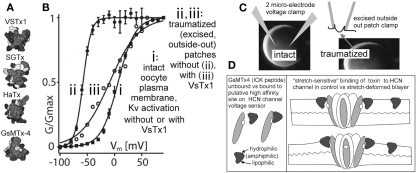
Figure 5.
Are there more MS voltage sensor toxins out there? (A) A collection of ICK peptides purified from tarantula venom (modified from Jung et al., 2005). The top 3 are established voltage sensor toxins, and the bottom one, GsMTx4, might be too (micromolar level GsMTx4 inhibits unidentified MS cation channels in astrocytes, but that is not the effect of interest here. GsMTx4 has powerful cardiac actions at <200 nm; Bode et al., 2001). (B) The “silver bullet scenario” as described in the text. VsTx1 action is mechanosensitive in that it inhibits this species of Kv channel (right-shifts activation; ii → iii) only when bilayer organization has been disrupted. The irreversible left-shift (i → ii) upon excision/trauma is as seen for some Nav channels (Figure 4), and like the advent of toxin efficacy, is ascribed to, (C) the difference in bilayer mechanics between the two recording conditions. (D) how GsMTx4 might act as a stretch-sensitive toxin for HCN channel voltage sensors. The scenario at left mirrors known behavior of VsTx1 and SGTx on their targets (Kv voltage sensors) and that at right suggests how membrane stretch might increase the inhibitory efficacy of GsMtx4. It is also possible to imagine membrane deformations that decreased the affinity of amphiphilic voltage sensor toxins for their targets. HCN channels are mentioned here, but the idea would apply for any voltage sensor, even for stand-alone ones (see Figure 1C; proton channels, voltage sensitive phosphatases as described, e.g., in Krepkiy et al., 2009).