Abstract
Lymphocyte subsets, activation markers and apoptosis were assessed in 20 HIV-exposed noninfected (ENI) children born to HIV-infected women who were or not exposed to antiretroviral (ARV) drugs during pregnancy and early infancy. ENI children and adolescents were aged 6–18 years and they were compared to 25 age-matched healthy non-HIV-exposed children and adolescents (Control). ENI individuals presented lower CD4+ T cells/mm3 than Control group (control: 1120.3 vs. ENI: 876.3; t-test, p = 0.030). ENI individuals had higher B-cell apoptosis than Control group (Control: 36.6%, ARV exposed: 82.3%, ARV nonexposed: 68.5%; Kruskal–Wallis, p < 0.05), but no statistical difference was noticed between those exposed and not exposed to ARV. Immune activation in CD4+ T, CD8+ T and in B cells was comparable in ENI and in Control children and adolescents. Subtle long-term immune alterations might persist among ENI individuals, but the clinical consequences if any are unknown, and these children require continued monitoring.
Keywords: Human Immunodeficiency Virus, apoptosis, lymphocyte activation, antiretroviral agents, CD4 T cell, B cell
Introduction
The use of highly active antiretroviral therapy (HAART) has decreased the vertical transmission of HIV-1 infection to rates as low as 0.99% [1], permitting the birth of many HIV-exposed noninfected (ENI) children. Nowadays, the concern is whether HIV and/or antiretroviral exposure could cause long-term consequences to those children and adolescents.
A few previous studies have addressed this issue [2–8], highlighting the state of immune activation [3, 5, 7, 8] in children born to HIV-infected mothers. Other studies have shown that infants who were exposed to nucleoside reverse transcriptase inhibitors (NRTIs) had mitochondrial dysfunction [9].
In a cohort of Latin American and Caribbean ENI, risk of infection early in life was associated with maternal CD4+ count between 200 and 500 cells mm−3, receipt of antibiotics in the peripartum period and country of origin [10]. Recently, a case series of eight ENI children with unusual or severe infections was reported in South Africa [11]. All eight infants received full mother-to-child transmission (MTCT) prophylaxis consisting of maternal single dose nevirapine plus zidovudine from at least 34 weeks, plus neonatal single dose nevirapine plus 7 days zidovudine, except one who received only neonatal zidovudine. Fourteen episodes of primary infections and four nosocomial infections, including Pneumocystis jiroveci pneumonia (n = 3), cytomegalovirus colitis with perforation (n = 1), Pseudomonas sepsis (n = 2), hemorrhagic varicella (n = 1), group A streptococcus meningitis and endocarditis (n = 1) were identified in these children. Interestingly, low CD4+ T cells and B-cell depletion were noticed in some of them.
Numbers of ENI children are increasing worldwide as a consequence of effective MTCT interventions for pregnant HIV-infected women and their offspring. Taking into consideration the different reports mentioned above, the aim of this study was to evaluate lymphocyte subsets, their activation markers and spontaneous apoptosis in HIV-exposed noninfected children and adolescents who were or not exposed to antiretroviral drugs during pregnancy and early infancy.
Material and Methods
Patients and study design
The protocol was approved by the Ethics Committee of the Federal University of São Paulo, in São Paulo, Brazil. All parents gave written informed consent prior to enrollment in the study. From January 2006 to August 2007, we evaluated 20 ENI aged 6 to 18 years and 25 healthy non-HIV-exposed and seronegative age-matched children and adolescents (control group: Control). All ENI children and adolescents were followed at the Pediatric AIDS Outpatient Clinic of the Federal University of São Paulo in São Paulo, Brazil. In utero and perinatal HIV exposure was based on maternal HIV infection diagnosis performed before pregnancy or at delivery of those children. Information on maternal antiretroviral treatment during pregnancy and delivery and on neonate antiretroviral prophylaxis was collected from the ENI records and/or maternal interview. Among the mothers who received antiretroviral drugs (n = 11), six received zidovudine alone, four with lamivudine or didanosine and one received different combinations of drugs, including zidovudine, lamivudine, nevirapine, indinavir and ritonavir. All children born to those women received zidovudine for 6 weeks.
Children and adolescents were considered HIV-exposed noninfected if they had two negative HIV–RNA detection assays, with the second test performed after 4 months of age; alternatively, if diagnosis was performed after 18 months of age, they should have negative HIV serological assays [12].
Inclusion criteria for ENI were only based on age (6–18 years), a clear in utero/perinatal HIV exposure and exclusion of HIV infection. Selection was not based on the presence or absence of chronic or previous serious diseases.
Children and adolescents from Control group were selected at a general pediatric outpatient clinic in São Paulo. They were born to healthy mothers after uneventful pregnancies and they did not refer any previous serious disease. At assessment, they did not present any diseases and were not on any medication. All of them were tested for HIV serology and they were all seronegative.
Blood collection
Five milliliters of blood were drawn by venous puncture for complete blood count (CBC), phenotypic analysis of peripheral blood mononuclear cell subsets using the flow cytometry assays and apoptosis assessment.
Complete blood count
All samples were tested using Advia 120 automatic counter (Bayer, Dublin and Ireland) and confirmed by a slide smear performed for manual count.
Flow cytometry analyses
Peripheral blood mononuclear cells were assessed by four-color flow cytometry (FACSCalibur, BD Biosciences, Franklin Lakes, USA) after cell staining using lyse-wash protocol and analyzed using the CellQuest software (BD Biosciences, San Jose, USA). Isotypic controls (IgG1-FITC, IgG1-PE, IgG1-APC, IgG2a-PE, all from BD Biosciences, San Jose, USA) were used to evaluate nonspecific staining. The number of cells per cubic milliliter of blood was obtained using the lymphocyte counts from the CBC.
Cell subsets
CD4+ and CD8+ T lymphocytes. The markers used to assess the subpopulation within the CD4+ (CD3-APC and CD4-PerCP labeled) and CD8+ (CD3-APC and CD8-PerCP labeled) populations were CD45RA-FITC and CCR7-PE (BD, San Jose, USA). In both CD4+ and CD8+ populations, the ‘naïve’ cells were CD45RA+CCR7+. The ‘central memory’ cells were CD45RA-CCR7+ and the ‘effector memory’ cells were CD45RA–CCR7–. The ‘terminally differentiated memory’ cells, more abundantly seen among CD8+ cells than in the CD4+ cohort, were CD45RA+CCR7– [13, 14].
CD4+ and CD8+ T lymphocyte activation
Lymphocyte activation was evaluated by quantification of CD38 molecules on CD4+ T cells, CD8+ T cells and on B cells using CD38 QuantiBRITE (BD, San Jose, USA).
NK cell
NK cells were quantified by the CD45+CD3–CD56+CD16+ phenotype.
B cells
B lymphocytes were identified as CD3–CD19+ cells and their maturation subsets were evaluated using CD27–FITC. Naïve B cells were CD3–CD19+CD27– and memory B cells were CD3–CD19+CD27+.
Spontaneous apoptosis evaluation was performed using active caspase-3-PE monoclonal antibody. Blood samples were diluted 1:10 in RPMI 1640 medium supplemented with l-glutamine (2 mM), penicillin (100.000 UI l–1) and streptomycin (100.000 µg l–1) (culture medium) (Gibco, New York, USA), and then incubated at 37°C for 18–20 h. After that, the cells were washed, lysed and the staining was done according to the manufacturer’s protocol (BD). The analysis was performed gating lymphocytes, and then evaluating T and B cells in regard to intracellular caspase-3 marking. For that analysis, 10 000 events were collected in the lymphocyte gate.
Statistical analysis
Data were analyzed using MINITAB 14 (Minitab, State College, USA) and Stata 7.0 (Stata, College Station, USA). Group characteristics at study entry were compared using t-test and Chi-squared test. For peripheral blood leukocytes, phenotype analyses and apoptosis analyses, t-test was performed. For apoptosis analysis between Control, ENI with ARV exposure (ENI: ARV exposure) and ENI without ARV exposure (ENI: No ARV exposure), Kruskal–Wallis was used. Logarithmic transformation was done when necessary in order to normalize the distribution. Level of significance was set at p < 0.05.
Results
Characteristics of study subjects
Control and ENI groups were comparable in respect to age (mean age ± SD: Control, 10 ± 4.0 years; ENI, 9 ± 1.9 years, t-test, p = 0.105) and gender (Control, 53% female; ENI, 50% female, Chi-squared test, p = 0.864). None of the Control or ENI individuals reported any previous serious disease or hospitalization; also, none of them reported any intercurrent illness preceding or at assessment. Neither Control nor ENI individuals were using steroids or immunosuppressive drugs at enrolment.
CD3+, CD4+ and CD8+ T cells, B cells, NK cells and maturation subsets
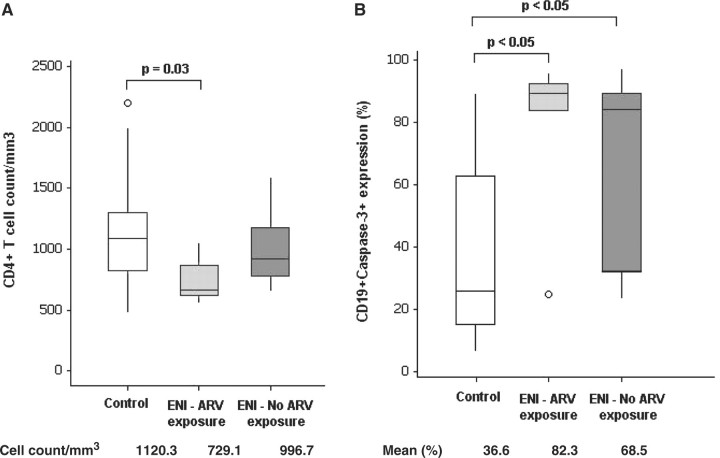
CD4+ T-cell counts were lower in ENI individuals than in Control individuals (Table 1). When ENI children and adolescents were separated according to antiretroviral exposure during pregnancy and early infancy, only ENI group with ARV exposure had significantly lower CD4+ T-cell counts than Control group (Fig. 1A). No differences between Control and ENI groups were identified for CD8+ T cells, B cells or NK cells.
Table 1.
Absolute values and percentages of lymphocyte subsets, number of CD38 molecules/cell and apoptosis level on lymphocytes in Control and ENI group.
| Cell subsets | Control (n = 25) | ENI (n = 20) | p-value |
|---|---|---|---|
| CD4+ T cells (cells mm–3) | 1120.3 ± 450.3 (476.8–2197.5) | 876.3 ± 270.8 (552.7–1593.1) | 0.030† |
| Naïve: CD45RA+CCR7+ (%) | 48.6 ± 7.8 (31.8–64.0) | 48.1 ± 8.4 (32.5–61.1) | 0.863† |
| Central memory: CD45RA-CCR7+ (%) | 22.4 ± 5.5 (13.8–31.9) | 20.9 ± 6.1 (5.9–30.0) | 0.419† |
| Peripheral memory: CD45RA-CCR7– (%) | 20.1 ± 6.3 (8.0–39.7) | 22.5 ± 7.4 (6.2–38.0) | 0.338† |
| Terminally differentiated: CD45RA+CCR7− (%) | 8.9 ± 5.6 (3.5–31.3) | 8.5 ± 6.6 (1.2–32.2) | 0.343‡ |
| CD38 molecules/cell | 3162.5 ± 1268.9 (1256.9–7278.4) | 3294.2 ± 1104.9 (1767.1–5039.8) | 0.586† |
| CD4+Caspase-3+ (%) | 2.9 ± 1.9 (0.6–8.1) | 4.2 ± 2.7 (1.4–11.0) | 0.080† |
| CD8+ T cells (cells mm−3) | 635.7 ± 303.5 (210.7–1400.6) | 658.4 ± 587.8 (200.9–2961.3) | 0.417‡ |
| Naïve: CD45RA+CCR7+ (%) | 40.5 ± 14.6 (15.9–67.8) | 40.9 ± 11.8 (11.5–64.4) | 0.969† |
| Central memory: CD45RA–CCR7+ (%) | 3.3 ± 1.6 (1.1–8.7) | 2.5 ± 1.2 (1.1–6.3) | 0.053† |
| Peripheral memory: CD45RA-CCR7– (%) | 29.2 ± 8.4 (13.0–48.2) | 27.3 ± 13.8 (4.8–71.6) | 0.486‡ |
| Terminally differentiated: CD45RA+CCR7– (%) | 27.0 ± 10.4 (6.9–48.2) | 29.2 ± 12.1 (8.2–51.6) | 0.585† |
| CD38 molecules/cell | 1773.1 ± 812.7 (705.5–3438.3) | 2491.7 ± 3206.6 (710.6–15706.6) | 0.607‡ |
| CD8+Caspase-3+ (%) | 3.6 ± 2.8 (0.9–11.5) | 2.9 ± 2.0 (0.9–9.8) | 0.602† |
| CD19+ cells (cells mm–3) | 396.1 ± 207.0 (127.5–980.3) | 401.4 ± 142.3 (147.9–701.0) | 0.847† |
| Naïve: CD19+CD27– (%) | 79.0 ± 8.7 (57.8–90.9) | 80.2 ± 6.8 (67.3–90.7) | 0.609† |
| Memory: CD19+CD27+ (%) | 21.0 ± 8.7 (9.1–42.2) | 19.8 ± 6.8 (9.3–32.7) | 0.609† |
| CD38 molecules/cell | 3048.6 ± 3139.5 (1222.8–17714.4) | 2885.6 ± 1238.6 (1078.0–5897.0) | 0.444‡ |
| CD19+Caspase-3+ (%) | 36.6 ± 27.7 (6.3–89.4) | 75.4 ± 26.4 ± (23.6–97.4) | <0.001† |
| NK cells (cells mm–3) | 277.4 ± 144.5 (91.5–676.7) | 290.5 ± 106.4 (60.9–466.9) | 0.813† |
Data are reported as mean ± SD and range in parenthesis. †t-test. ‡Mann–Whitney.
Fig 1.
Boxplot of number of CD4+ T cells mm−3 (A) and caspase-3 expression on B lymphocyte (B) in healthy control group, ENI group who were exposed to antiretroviral drugs (ENI: ARV exposure) during pregnancy and after birth and ENI group who were not exposed to antiretroviral drugs (ENI – no ARV exposure) during pregnancy or after birth. Comparison between groups in respect to CD4+ T-cell count was performed with ANOVA, with multiple comparisons performed with Tukey’s test; Caspase-3 expression on B cells was evaluated with Kruskal–Wallis test, with multiple comparisons performed with Dunn’s test.
T- and B-cell maturation subsets were comparable between Control and ENI individuals (Table 1).
Quantification of CD38 molecules on T and B cells
Mean CD38 molecules on T cells and B cells were similar in Control and ENI groups (Table 1).
Apoptosis evaluation
ENI group had higher mean percentages of B cells in apoptosis than Control group (Table 1). When ENI children and adolescents were separated according to antiretroviral exposure during pregnancy and early infancy, no statistically significant differences were noticed between ENI subgroups in respect to B cells apoptosis (Fig. 1B). However, a lower variability and a tendency to elevated percentages of apoptotic cells was noted among individuals from ENI group who were exposed to antiretroviral drugs when compared with ENI who were not exposed to antiretroviral drugs (Fig. 1B).
Discussion
We have shown that older vertically ENI children and adolescents have lower CD4+ T-cell counts when compared to healthy control children, but still within reference values for age [15]. They present with high apoptosis levels in B cells when compared to healthy age-matched controls. In spite of no statistical difference, percentages of apoptosis for B lymphocytes were higher in ARV-exposed group than nonexposed group. Immune activation as assessed by CD38 expression was not noticed in ENI children and adolescents.
Despite normal distribution of naïve and memory CD4+ T cells, there was a decrease in absolute CD4+ T cells in ENI as compared to Control children and adolescents. An earlier study [6] also found lower CD4+ T-cell counts in cord blood samples from ENI children when compared to a Control group. The authors also observed that ENI children had reduced thymic output as low frequencies of CD4+ T cells with T-cell receptor excision circles (TRECs) were found when compared with controls. That led them to hypothesize that low CD4+ T-cell levels might be due to impaired progenitor cell function. We did not evaluate thymic function but it is possible that thymus output remains persistently reduced in ENIs as compared to Controls.
Clinical evidence of immunodeficiency was recently observed in eight ENI infants from an African cohort sent for exclusion of primary immunodeficiency at Tygerberg Children’s Hospital in South Africa [11]. It was noticed that four of them presented reduced CD4+ and/or CD8+ and/or CD19+ lymphocytes. Of note, one infant had persistent B-cell depletion of unknown etiology that resulted on maintained intravenous immunoglobulin therapy.
An imbalance of naïve and memory CD4+ and CD8+ T lymphocytes [8] and a higher state of immune activation [3, 5, 7, 8] have been described in neonates, infants and young children. In contrast, older ENI children and adolescents from our study had percentages of naïve and memory CD4+ and CD8+ T cells similar to controls. Also, no evidence of immune activation was observed on T or B cells. It is possible that if abnormalities in these parameters are present in infancy, the distribution of maturation markers normalizes as ENI get older and that they have a progressive decay of immune activation.
When evaluating apoptosis in different lymphocyte subpopulations, we found an increase of B-lymphocyte apoptosis in ENI. That was noticed even without perinatal exposure to ARV drugs. Though not statistically significant, ARV exposure seemed to result in higher levels of lymphocyte apoptosis than without ARV exposure.
There are no previous reports on B-lymphocyte apoptosis in ENI population. ENI neonates have been shown to have higher T-cells apoptosis levels than healthy control neonates, but no previous studies have investigated apoptosis in lymphocytes from older vertically ENI individuals [4]. Velilla et al. [16] reported that ENI adults had higher spontaneous apoptosis on monocytes than control group, and they suggested that might be a mechanism of protection against HIV infection.
In HIV infection, apoptosis seems to be induced preferentially via death receptors (CD95 and TNFR) [17–19]. The use of highly active antiretroviral therapy (HAART) reduces viral replication, increases CD4+ T-cell counts and decreases immune activation and apoptosis in HIV-infected individuals [18, 20, 21]. Herbeuval et al. [22] showed that HAART also leads to a decrease of death receptor ligand expression such as FasL and DR5. However, HAART was ineffective in reducing death receptors expression such as Fas and TRAIL. One could speculate that co-infection or re-activation of latent non-HIV viruses could induce apoptotic ligands that might result in apoptosis of cells that persistently express DR5. Alternatively, mitochondrial toxicity could be involved, as discussed below. The mechanism for the increased B-cell apoptosis remains to be further explored.
It is known that NRTIs can induce mitochondrial dysfunction because they have affinity for mitochondrial gamma DNA polymerase [23, 24]. Scruggs and Naylor [25] suggested that mitochondrial toxicity due to zidovudine administration may be caused by mitochondrial DNA (mtDNA) depletion, direct effects on mitochondrial bioenergetics, oxidative stress and reduced content of L-carnitine. All those factors could lead to apoptosis. Although mitochondrial DNA depletion is reversible by discontinuing NRTIs, it is not always to normal levels [26].
In ENI individuals, some reports have shown evidence of mitochondrial toxicity associated with ARV exposure [27, 28]. It is possible that, similarly to what happens to HIV-infected patients who discontinue ARV drugs, ENI individuals might also persist with low mtDNA numbers, maintaining high apoptosis levels after interruption of HIV prophylactic medication.
In summary, the findings in ENI individuals are indicative of long-term effects of either in utero exposure to HIV and also possibly to antiretroviral drugs they have received during embryonic and fetal development, as well as in early infancy. They can be definitely distinguished from healthy control individuals even when they reach adolescence. Lower CD4+ T-cell counts and a persistent apoptosis of B cells are of special relevance. The few but worrying reports of ENI children with clinical symptoms possibly linked to immune alterations reinforce the need of further studies in the ENI population that is increasing in number and advancing in age.
Funding
Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) – Brazil (04/15316-4, 04/15317-0); Miami Fogarty (AITRP5D43TW000017); Miami Developmental Center for AIDS Research (P30AI073961).
References
- 1.European Collaborative Study. Mother-to-child transmission of HIV infection in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40:458–65. doi: 10.1086/427287. [DOI] [PubMed] [Google Scholar]
- 2.Gesner M, Papaevangelou V, Kim M, et al. Alteration in the proportion of CD4 T lymphocytes in a subgroup of human immunodeficiency virus-exposed-non-infected children. Pediatrics. 1994;93:624–30. [PubMed] [Google Scholar]
- 3.Rich KC, Siegel JN, Jennings C, et al. Function and phenotype of immature CD41 lymphocytes in healthy infants and early lymphocyte activation in uninfected infants of human immunodeficiency virus-infected mothers. Clin Diag Lab Immunol. 1997;4:358–61. doi: 10.1128/cdli.4.3.358-361.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Economides A, Schmid I, Anisman-Posner DJ, et al. Apoptosis in cord blood T lymphocytes from infants of human immunodeficiency virus-infected mothers. Clin Diagn Lab Immunol. 1998;5:230–4. doi: 10.1128/cdli.5.2.230-234.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clerici M, Saresella M, Colombo F, et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood. 2000;96:3866–71. [PubMed] [Google Scholar]
- 6.Nielsen SD, Jeppesen DL, Kolte L, et al. Impaired progenitor cell function in HIV-negative infants of HIV-positive mothers results in decreased thymic output and low CD4 counts. Blood. 2001;98:398–404. doi: 10.1182/blood.v98.2.398. [DOI] [PubMed] [Google Scholar]
- 7.Van Rie A, Madhi SA, Heera JR, et al. Gamma interferon production in response to Mycobacterium bovis BCG and Mycobacterium tuberculosis antigens in infants born to human immunodeficiency virus-infected mothers. Clin Vaccine Immunol. 2006;13:246–52. doi: 10.1128/CVI.13.2.246-252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ono E, Nunes dos Santos AM, de Menezes Succi RC, et al. Imbalance of naive and memory T lymphocytes with sustained high cellular activation during the first year of life from uninfected children born to HIV-1-infected mothers on HAART. Braz J Med Biol Res. 2008;41:700–8. doi: 10.1590/s0100-879x2008000800011. [DOI] [PubMed] [Google Scholar]
- 9.El Beitune P, Duarte G, Quintana SM, et al. Antiretroviral therapy during pregnancy and early neonatal life: consequences for HIV-exposed, uninfected children. Braz J Infect Dis. 2004;8:140–50. doi: 10.1590/s1413-86702004000200004. [DOI] [PubMed] [Google Scholar]
- 10.Mussi-Pinhata MM, Freimanis L, Yamamoto AY, et al. Infectious disease morbidity among young HIV-1-exposed but uninfected infants in Latin American and Caribbean countries: the National Institute of Child Health and Human Development International Site Development Initiative Perinatal Study. Pediatrics. 2007;119:e694–704. doi: 10.1542/peds.2006-1856. [DOI] [PubMed] [Google Scholar]
- 11.Slogrove AL, Cotton MF, Esser MM. Severe infectious in HIV-exposed uninfected infants: clinical evidence of immunodeficiency. J Trop Pediatr. 2009 doi: 10.1093/tropej/fmp057. Jul 14 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 12. American Academy of Pediatrics. Human immunodeficiency virus infection. In: Pickering LK (ed). 2006 Red Book: Report of the Committee on Infectious Diseases, 27th edn. Elk Grove Village: American Academy of Pediatrics, 2006;385–9. [Google Scholar]
- 13.Harari A, Rizzardi GP, Ellefsen K, et al. Analysis of HIV-1- and CMV-specific memory CD4 T-cell responses during primary and chronic infection. Blood. 2002;100:1381–7. doi: 10.1182/blood-2001-11-0080. [DOI] [PubMed] [Google Scholar]
- 14.Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101:4260–6. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 15.Shearer WT, Rosenblatt HM, Gelman RS, et al. Pediatric AIDS Clinical Trials Group. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112:973–80. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Velilla PA, Hoyos A, Rojas M, et al. Apoptosis as a mechanism of natural resistance to HIV-1 infection in an exposed but uninfected population. J Clin Virol. 2005;32:329–35. doi: 10.1016/j.jcv.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Clerici M, Sarin A, Henkart PA, et al. Apoptotic cell death and cytokine dysregulation in human immunodeficiency virus infection: pivotal factors in disease progression. Cell Death Differ. 1997;4:699–706. doi: 10.1038/sj.cdd.4400314. [DOI] [PubMed] [Google Scholar]
- 18.Gougeon ML, Lecoeur H, Sasaki Y. Apoptosis and the CD95 system in HIV disease: impact of highly active anti-retroviral therapy (HAART) Immunol Lett. 1999;66:97–103. doi: 10.1016/s0165-2478(98)00167-9. [DOI] [PubMed] [Google Scholar]
- 19.De Oliveira Pinto LM, Garcia S, Lecoeur H, et al. Increased sensitivity of T lymphocytes to tumor necrosis factor receptor 1 (TNFR1)- and TNFR2-mediated apoptosis in HIV infection: relation to expression of Bcl-2 and active caspase-8 and caspase-3. Blood. 2002;99:1666–75. doi: 10.1182/blood.v99.5.1666. [DOI] [PubMed] [Google Scholar]
- 20.Plana M, Martínez C, García F, et al. Immunologic reconstitution after 1 year of highly active antiretroviral therapy, with or without protease inhibitors. J Acquir Immune Defic Syndr. 2002;29:429–34. doi: 10.1097/00126334-200204150-00001. [DOI] [PubMed] [Google Scholar]
- 21.Plana M, Ferrer E, Martínez C, et al. Immune restoration in HIV-positive, antiretroviral-naive patients after 1 year of zidovudine/lamivudine plus nelfinavir or nevirapine. Antivir Ther. 2004;9:197–204. [PubMed] [Google Scholar]
- 22.Herbeuval JP, Nilsson J, Boasso A, et al. HAART reduces death ligand but not death receptors in lymphoid tissue of HIV-infected patients and simian immunodeficiency virus-infected macaques. AIDS. 2009;23:35–40. doi: 10.1097/QAD.0b013e32831cb907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis W, Dalakas MC. Mitochondrial toxicity of antiviral drugs. Nat Med. 1995;1:417–22. doi: 10.1038/nm0595-417. [DOI] [PubMed] [Google Scholar]
- 24.Saitoh A, Fenton T, Alvero C, et al. Impact of nucleoside reverse transcriptase inhibitors on mitochondria in human immunodeficiency virus type 1-infected children receiving highly active antiretroviral therapy. Antimicrob Agents Chemother. 2007;51:4236–42. doi: 10.1128/AAC.00893-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scruggs ER, Naylor AJD. Mechanisms of zidovudine-induced mitochondrial toxicity and myopathy. Pharmacology. 2008;82:83–8. doi: 10.1159/000134943. [DOI] [PubMed] [Google Scholar]
- 26.McComsey GA, Paulesen DM, Lonergan JT, et al. Improvements in lipoatrophy, mitochondrial DNA levels and fat apoptosis after replacing stavudine with abacavir or zidovudine. AIDS. 2005;19:15–23. doi: 10.1097/00002030-200501030-00002. [DOI] [PubMed] [Google Scholar]
- 27.Blanche S, Tardieu M, Rustin P, et al. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet. 1999;354:1084–9. doi: 10.1016/S0140-6736(99)07219-0. [DOI] [PubMed] [Google Scholar]
- 28.Rizza SA, Badley AD. HIV protease inhibitors impact on apoptosis. Med Chem. 2008;4:75–9. doi: 10.2174/157340608783331443. [DOI] [PMC free article] [PubMed] [Google Scholar]