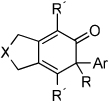
Table 2.
Ni-Catalyzed Cycloaddition of Diynes and Ketenesa
| Entry | Diyne | Ketene | Product | Yieldbc |
|---|---|---|---|---|
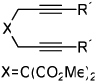 |
 |
 |
||
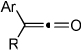
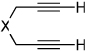
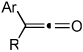
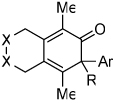
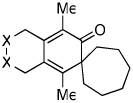
| 1 | 1 (R′ = Me) | a Ar= Ph R=Et | 1a | 82% |
| 2 | 1 | b Ar= Ph R=Ph | 1b | 46% |
| 3 | 1 | c Ar= Ph R=i-Pr | 1C | 50% |
| 4 | 2 (R′ = Ph) | a Ar= Ph R=Et | 2a | 65% |
 |
||||
| 5 | 3 (X=C(CO2Et)2) | a Ar= Ph R=Et | 3a | 54% |
| 6 | 4 (X=CH2) | a Ar= Ph R=Et | 4a | 35% |
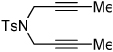 |
||||
| 7 | 5 | a Ar= Ph R=Et | 5a | 50% |
| 8 | 5 | d Ar= Ph R=n-Pr | 5d | 55% |
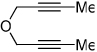 |
||||
| 9 | 6 | a Ar= Ph R=Et | 6a | 33% |
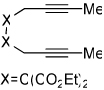 |
 |
 |
||
| 10 | 7 | a Ar= Ph R=Et | 7a | 91% |
| 11 | 7 | e Ar= p-OMe-Ph R=Et | 7e | 81% |
| 12 | 7 | f Ar= p-Me-Ph R=Et | 7f | 80% |
| 13 | 7 | g Ar= p-F-Ph R=Et | 7g | >99%c |
| 14 | 7 | h Ar= Ph R=Me | 7h | 65% |
| 15 | 7 | d Ar= Ph R=n-Pr | 7d | 76% |
 |
 |
|||
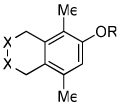
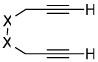
| 16 | 7 | i | 7i-i' | 82% |
 |
 |
|||
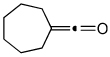
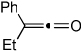
| 17 | 7 | j | 7j | 76% |
 |
 |
 |
||
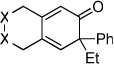
| 18 | 8 (X=C(CO2Et)2) | a | 8a | 78% |
| 19 | 9 (X= CH2) | a | 9a | 33% |
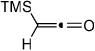
Reaction conditions: 5 mol% Ni(COD)2, 5 mol% DPPB, diyne (1 equiv, 0.1 M), ketene (1.2 equiv) in toluene at 60 °C, 5 h.
Isolated yields.
Average of at least two runs.
Crude ketene was used.
