Abstract
Some living organisms produce visible light (bioluminescence) for intra- or interspecific visual communication. Here, we describe a remarkable bioluminescent adaptation in the marine snail Hinea brasiliana. This species produces a luminous display in response to mechanical stimulation caused by encounters with other motile organisms. The light is produced from discrete areas on the snail's body beneath the snail's shell, and must thus overcome this structural barrier to be viewed by an external receiver. The diffusion and transmission efficiency of the shell is greater than a commercial diffuser reference material. Most strikingly, the shell, although opaque and pigmented, selectively diffuses the blue-green wavelength of the species bioluminescence. This diffusion generates a luminous display that is enlarged relative to the original light source. This unusual shell thus allows spatially amplified outward transmission of light communication signals from the snail, while allowing the animal to remain safely inside its hard protective shell.
Keywords: bioluminescence, signal dispersion, biophotonics, light manipulation, shell adaptation, wavelength-specific diffusion
1. Introduction
Bioluminescence—the production of visible light by organisms—is usually adapted for furthest possible transmission in the surrounding environment by modulation of the colour and/or display of light [1–4]. Known functions associated with light production encompass attracting mates or prey [5–7], detecting prey [8–10], counter-shading camouflage [11–14] and deterring predators [15–18]. All of these functions are well documented in the phylum Mollusca [19,20], although the literature is dominated by the highly specialized organs, spectacular display and complex behaviour of pelagic cephalopods [21–25].
In organisms where light production is internal, the light signal must traverse biological material (e.g. epithelium or scales) in order to be visible from the outside. This emitted bioluminescent light may be transmitted, absorbed, reflected or diffused by the biological material. When thin and transparent, the emitted light may simply transmit directly through [26,27]. Absorption of light is especially common in organisms where the bioluminescence appears as a glow, either from symbiosis with luminous bacteria or from an intrinsic photophore. There, the use of heavily pigmented light-absorbing tissue can be used like a shutter over the light organ. If the pigments can modify the spectral nature of the emitted light, the organism can control its light output in terms of intensity and/or colour [28–30]. Reflection of light may be more important in organisms where the bioluminescence light is preferentially emitted in one direction (e.g. downward or upward, or towards a specific opening of the light organ and optimized by redirection [31–33]).
If light is scattered (or diffused) away from its source, the mechanism may involve a combination of the above scenarios. Indeed, diffusion often involves light-guiding structures, which can channel light away from its source. Such diffusion may involve the development of reflecting surfaces or the transformation of otherwise opaque tissues into transparent, light-guiding material. For example, some cephalopod photophores may be surrounded by bundles of collagen fibres that form an almost hemispherical system of light-guides; these use internal reflection to redirect light to a broader emission area [29,32,34]. The use of more complex light-channelling structures is found in some species of fish, where light is distributed through bone, muscle, bladder or connective tissue to an area larger than its source [8,35–38]. Even more complex rod-like structures can channel light in a cephalopod, although details on whether these structures actually enable spatial amplification of signals is unknown, given that these particular photophores rarely luminesce [39]. To our knowledge, there is no report of calcified structures involved in diffusion and spatial modification of a bioluminescent signal.
In contrast to the large literature on bioluminescence in the pelagic zone, reports of bioluminescence for benthic marine molluscs are much less common [9], and the systems, mainly subject to anecdotal reports, are rarely fully characterized (but see literature on bentho-pelagic Euprymna). In caenogastropods, there is only one unconfirmed report of bioluminescence in a species of Tonna [40], and confirmed reports for several species in the family Planaxidae [41]. Initial attempts to characterize bioluminescence in Planaxis labiosus and Hinea brasiliana suggested that blue-green bioluminescence of an unknown wavelength was produced by artificial agitation, and that the light was produced intracellularly in multiple species [41,42].
Here, we fully characterize bioluminescence in H. brasiliana, an intertidal marine snail in the Planaxidae, a family that contains approximately 20 species in six genera [43]. Reports of light production are available for three of the genera, namely Planaxis [41], Angiola [43] and the one studied here, Hinea [42]. We describe a unique mechanism of spatially amplifying a bioluminescent signal using a hard, calcified shell for diffusion. Indeed, we demonstrate that the shell of the snail acts as a unique diffuser that propagates the specific wavelength of the bioluminescence, causing the light signal to appear enlarged to the receiver.
2. Material and methods
Adult individuals (0.5–1.5 cm shell length) of H. brasiliana (Lamarck, 1822) were collected by hand at low tide under rocks from Merry Beach and Hastings Point, NSW, Australia. Photonic properties of the bioluminescence were measured directly from the body of a snail, after shell removal using a rotary circle cutter (400 series XPR Dremel, Wisconsin, USA). Bioluminescence was experimentally stimulated with potassium chloride (KCl, 200 mM final concentration), which is commonly used on bioluminescent invertebrates to depolarize tissues and trigger light production until exhaustion of their luminous constituents [44]. This treatment usually allows enough light to be produced for photonic characterization. The spectrum of the light production was recorded every second, following 1 s integration of emitted light, using a low-light SE200 Echelle Spectrograph (Catalina Scientific, Arizona, USA). The intensity of the light production was measured for several minutes (depending on the experimental treatment) every 0.2 s, using a Sirius luminometer (Berthold Inc., Germany) for all experiments.
However, in order to describe the detailed kinetics of spontaneous and mechanically stimulated flashes, light measurements were also made every 0.01 s in an integrating light chamber, using a photon-counting Electron Tubes model P10 232 photomultiplier fitted with a Uniblitz electronic shutter (Vincent Associates, New York, USA). The number of photons emitted was then expressed as photons per 10 milliseconds on the basis of radiometric calibration with a 310 multispectral source (Optronics Laboratories, Florida, USA), as used to characterize short flashing patterns [45,46].
Bioluminescence was also assessed with an intact snail. However, the snail retracts deeply into its shell when manipulated and exposed to chemical stimulation. Such behaviour allows the snails to be hermetic to external stimulants, which failed to trigger light. In order to address this, two holes of about 1.5 mm were drilled (400 series XPR Dremel) through the ventral side of the second largest whorl of the shell (away from the side of light production), with the snail alive inside. This treatment rendered the snail ‘permeable’ to external chemical stimulants while keeping it deeply lodged in its shell. The snail was then placed in 200 µl of artificial sea water to which KCl (as described above) or the neuro-mediator acetylcholine (Ach, 1 mM final concentration) was added to stimulate light production, which in this case was recorded for 15 min. The spectrum of light production was also measured from such snails in their shells, using KCl as a stimulant (as described above).
The calcified shell was characterized for various photonic attributes, using a whole shell from either live or methanol-preserved specimens. In all cases, the soft mollusc body was retracted far into the shell. To avoid potential disruption to the shell, no physical or chemical attempts were made to remove it. We first tested whether the shell equally transmits (or absorbs) all wavelengths of light. The spectrum of light transmitted through the shell was measured by using two 600 µm-wide optic fibres, placed approximately 3 mm apart and in direct opposition (electronic supplementary material, figure S1); one emitted white light (tungsten–halogen calibrated, 300–1050 nm; Ocean Optics, Florida, USA), while the other received the transmitted light, and was connected to the spectrograph for spectrum profile identification. Measurements were made with the shell placed mid-distance between the fibres, and without the shell for a control. The emitting fibre was placed into the aperture of the shell, positioned about 1 mm away from the internal side as guided using a micromanipulator (Narishige, Japan).
For the transmission capacity (position a, electronic supplementary material, figure S1), the emitting/receiving fibre optics stayed aligned facing one another, and measurements from the different samples placed in between the two fibres were recorded under the same acquisition parameters (exposure time and gain). For the diffusion capacity (gradual lateral move from position a to b, electronic supplementary material, figure S1), the acquisition parameters from each sample were such that the light measured by the receiving fibre when facing the emitting fibre in position a was about 75 per cent of the optimal intensity range for the instrument, and the receiving fibre optic laterally and gradually (by millimetre steps) moved to position b (electronic supplementary material, figure S1) using the micro-manipulator for light measurements of the diffused light, without changing any acquisition parameters.
(a). Light transmission
We then analysed the shell for its light transmission capacity and compared it with standard Zenith diffuser material of thicknesses 100, 250 and 500 µm (SphereOptics, New Hampshire, USA). For comparison, the shell thickness was measured using an ocular micrometer on a Nikon SMZ1500 stereomicroscope. Light transmittance was quantified by using the same setting as above, with the two optic fibres placed in direct opposition. However, in this case, one emitted a set beam of blue-green light (522 ± 40 nm; Ocean Optics), which was observed to be the colour range of bioluminescence in this species [41], while the other received the transmitted light, and was connected to the spectrograph for quantification. We measured transmittance with no material placed between the fibres (100% control transmittance) and determined the exposure time that provided intensity values within the optimal resolution of the spectrograph. Keeping the settings constant, we repeated measurements with samples placed mid-distance between the fibres, as described above. Transmittance of the samples was then expressed relative to the initial full control transmittance with no sample.
(b). Light diffusion
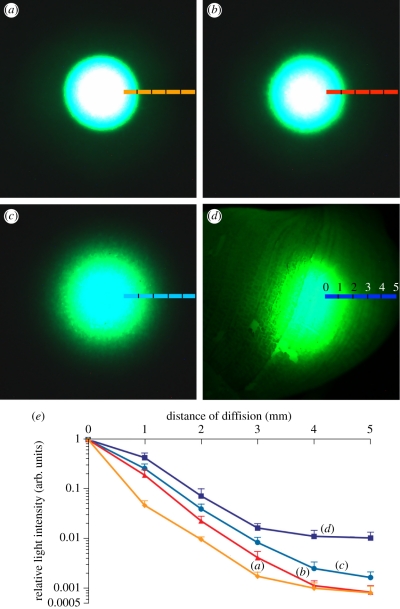
The shell was also analysed for its diffusion capacity and compared with the same three thicknesses of the standard Zenith diffuser material. We used the same settings as described above with the exception that the receiving optic fibre was operated with the micromanipulator to move lateral to the emitting fibre, at 1 mm intervals along a 5 mm transect (see transect lines in figure 3). At the termination of the transect, the emitting and receiving fibres were separated by 5 mm. At each millimetre point along the transect, the intensity of light was quantified by the spectrograph and expressed relative to the intensity of light directly transmitted when facing the emitting fibre light source (where transmittance was maximal). To accommodate any difference in transmittance among different samples, settings of the spectrograph were reset each time at 0 mm position so that the intensity values were in the maximal range of the instrument, and the setting kept constant along the transect. Micrographs of light diffusion were collected using a Nikon SMZ1500 stereomicroscope equipped with a cooled Q-Imaging Retiga 2000R digital camera controlled by Q-Capture Pro software (AG Heinze Inc., New York, NY, USA).
Figure 3.
Micrographs of light diffusion through standard Zenith diffuser of various thicknesses ((a) 100 µm; (b) 250 µm; (c) 500 µm), and the shell of H. brasiliana ((d) approx. 500 µm thick) under the same settings of illumination and photography. Coloured lines indicate transect along which light intensity was measured. White areas represent instrument saturation. (e) Intensity of diffused light expressed relative to transmittance where the light source is applied (= 0 mm) for millimetre steps away from the source (max. 5 mm, n = 6). Colour code corresponds to (a–d).
(c). Stimulation by other organisms
The natural stimulatory conditions and resulting bioluminescence profile of H. brasiliana were assessed experimentally through encounter experiments with a variety of other organisms that occur in the same habitat. In a first set of experiments, motile organisms (alive and freshly collected) were placed in a covered Petri dish containing artificial sea water and first observed by eye under dim ambient light to assess their general activity. The organisms were then categorized as either ‘high-contact’, whose motility could cause frequent and usually short contact events between organisms, or ‘low-contact’, whose low motility resulted in only rare and usually long contact events. High-contact organisms included the gammarid amphipod Cymadusa uncinata, the decapod prawn Palaemon macrodactylus and the polynoid polychaetes Arctonoe pulchra, all non-luminous species. Low-contact organisms consisted of other snails, either luminous conspecifics or local non-luminous species of Trochidae, Columbellidae, Muricidae and Nassariidae. The light production pattern was then quantified experimentally by including high- or low-contact organisms, or a combination, with H. brasiliana, using a Sirius luminometer (Berthold Inc., Germany) to record the emitted light every second for 1800 s (30 min).
In a second set of experiments, we specifically assessed whether the light production in H. brasiliana could be mechanically stimulated. This was tested by exposure to the actively swimming, non-predatory amphipod C. uncinata in a small Petri dish, allowing ample opportunities of chance contact to trigger light production. One H. brasiliana and one amphipod were placed together in a small dish where they could be separated by a translucent plastic divider. For each treatment (combined then separated by divider, or vice versa), the light production was recorded every 0.2 s for 15 min from the same paired individuals.
All experiments and measurements were completed with six to eight independent replicates, and box plots were used to represent the bioluminescence emitted by focal H. brasiliana under various experimental conditions. In each case, errors bars represent deciles (10th and 90th percentiles), boxes represent quartiles (25th, 50th, and 75th percentiles) and filled squares represent the means. Analysis of variance (ANOVA) was used to test the significance of differences between high- and low-contact organisms and background light, and between the shell and the various thicknesses of diffuser material in terms of distance of diffused light intensity. The original dataset was log(x + 1) transformed when heteroscedasticity occurred [47]. All statistical analyses were performed using Statview v. 5.0 software (SAS Institute, Inc.) with significance based on α of 0.05 for two-tailed comparisons. For sets of pairwise comparisons, p-values given in the text refer to the most conservative (i.e. least significant) among all significant pairwise values.
3. Results and discussion
(a). Origin and spectrum of emitted light
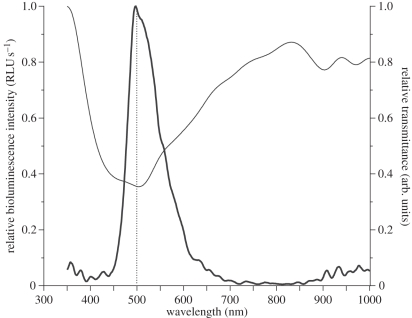
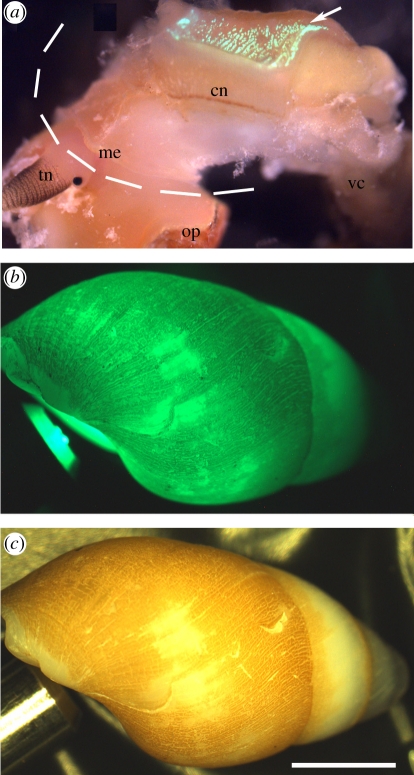
KCl-triggered bioluminescence from a snail body isolated from the shell (or from a snail retained with the shell, data not shown) occurred as a long-lasting unimodal glow with light intensity peaking within seconds of stimulation, followed by slow decay that could last up to 2 min. Emitted light (λmax 502 nm, with full width half max. of 80 nm; figure 1) was expressed in the blue-green range (480–520 nm) of the visible light spectrum, with emitted spectra remaining at similar wavelengths throughout the entire light production event (electronic supplementary material, figure S2). The epidermal cells producing light were autofluorescent only after production of bioluminescence (figure 2a). In agreement with observations made on P. labiosus [41], these cells appeared to correlate approximately with the location of the hypobranchial gland on the roof of the mantle cavity, and were organized in two patches on either side of the snail's body, posterior to the shell opening (figure 2a). This area of the body is fixed within the shell and cannot extend out of the shell aperture. Thus, in order to perform an ecological function, the bioluminescent signal of H. brasiliana must overcome the physical barrier of the shell to be visible from the outside.
Figure 1.
Instantaneous record of bioluminescence spectrum for an individual of H. brasiliana after stimulation with KCl 200 mM peaks at 502 nm (thick line). Transmittance of the visible white light spectrum through the shell material is minimal for that colour (thin line; measured as 1-absorbance, in arb. units), owing to wavelength-specific diffusion of the bioluminescent light. Dotted line marks 500 nm for reference.
Figure 2.
Hinea brasiliana. (a) Epidermis of an animal removed from its shell and showing intense fluorescence (arrow) after KCl-stimulation; dashed line represents shell edge. (b) Dark field view of shell containing retracted individual with blue-green light fibre-optic beam shone through shell aperture. (c) Same view in bright field. cn, ctenidium; me, mantle edge; op, operculum; tn, tentacle; vc, visceral complex. Scale bar, 2 mm.
(b). Spectral selectivity of the shell
Under natural light, the shell of H. brasiliana is opaque with a brown-yellow proteinaceous coating (periostracum) over the main whorl (figure 2c). Surprisingly, when shining a beam of white light into the aperture of the shell, most wavelengths of the light were transmitted directly through the shell quite efficiently (greater than 75%), except for the blue-green wavelengths (450–550 nm; figure 1). This photonic range overlaps with the specific wavelength of bioluminescence produced by this species, and the spectral peak of bioluminescent emission (approx. 500 nm, measured directly from the snail body) corresponds to the lowest peak of wavelength transmission through the shell (figure 1). This relationship suggests that the shell is adapted to selectively limit the direct transmission of the bioluminescence signal, and according to expectations under the Beer-Lambert Law should absorb the luminous signal and decrease its intensity. Paradoxically, we found that a discrete beam of blue-green light shone into the shell aperture (mimicking emitted bioluminescence) scattered these wavelengths efficiently to other parts of the shell otherwise not exposed to the original source and was emitted as a diffuse and spatially amplified light signal (figure 2b).
The wavelength of this diffused light was not altered despite passing through the shell's brown-yellow periostracum (data not shown). This relationship was observed in all cases, whether the animal was alive or dead, and preserved dried, in ethanol or methanol, despite the heterogeneities in colour and thickness of the periostracum. If the brown-yellow colour of the periostracum was influencing the transmissivity of the shell, we might expect the periostracum to absorb light of its complementary colour (blue-violet), which was not the case since this wavelength range was efficiently transmitted through the shell (figure 1). In addition, some living snails have had their periostracum eroded entirely from their shell and appear white, yet still show similar scattering capacity as those that retain a brown-yellow periostracum. Clearly, in H. brasiliana, the organic periostracum of the shell does not act in the same way as pigment-facilitated absorption or interference filters, as observed in some pelagic luminous fish [48] and squid [28,29]. In those studies, filters appear as a coloured layer of pigments that interfere with the bioluminescence emission spectrum and/or restrict the light spectrum to a narrower band width [30]. The mechanisms by which such wavelength-specific diffusion takes place in the shell of H. brasiliana still remain to be characterized, and are likely to be linked to the structural morphology of the calcium layers rather than the overlaying pigmented proteinaceous periostracum.
(c). Shell photonic transmission and diffusion capabilities
We measured the extent to which the shell was able to directly transmit blue-green light. When placed between a pair of source and receiver fibre optics, the shell (approx. 500 µm thick) directly transmitted only 0.63 per cent of the applied blue-green light source, the remaining 99.37 per cent being available for diffusion or absorption by the shell. From a biological standpoint, such direct transmittance appears rather low; yet from a physical standpoint, it remains unexpectedly high for such relatively thick material. Indeed, the transmittance is approximately eight times greater than for an equivalent 500 µm standard diffuser reference material (Zenith), which only transmitted 0.08 per cent of the light source. For comparison, thinner pieces (250 and 100 µm) of the same standard reference material transmitted 1.07 per cent and 1.78 per cent of the applied light, respectively. The high transmittance of blue-green wavelengths, which match those emitted from the underlying light organ, suggests that the shell still allows efficient transmittance capacity for the specific wavelengths of the bioluminescence.
An inverse relationship is usually expected between light transmittance and diffusion of a material, based on the fact that the greater the effective transmittance through the material, the less light remains available for scattering within the material. However, although the shell exhibited relatively high blue-green light transmittance when compared with a commercial diffuser, it still maintained an extraordinary capacity to diffuse light. Indeed, the discrete beam of artificial blue-green light propagated homogeneously to the entire shell (figure 2b), thus generating an illuminated diffuse area more than 10 times greater than a comparison to the 500 µm standard Zenith diffuser material (figure 3a–d). When quantified along a transect moving laterally away from the light source, the intensity of diffused light was always greater for the shell of H. brasiliana than for any thickness of the standard Zenith diffuser material (figure 3e). Starting at 1 mm distance from the point source of light, the intensity of diffused light was significantly greater for the shell compared with the 100 µm (p < 0.0106) and 250 µm (p < 0.0377) diffuser reference material, while the difference with respect to the 500 µm reference material gained significance at 4 mm (p < 0.0041; figure 3e). Beyond 3 mm from the source, only light from the shell sample was clearly visible by eye (although all samples remained detectable and measurable with our instrumentation). At 5 mm, the shell showed intensities of diffused light about 10 times greater than the comparable 500 µm standard Zenith material (figure 3e).
This remarkable diffusion capacity allows the snail to use the small, discrete sources of bioluminescent light it produces to generate spatially larger signals to the exterior. The light diffusion through the shell was not linked to the gross architecture or curvature of the shell since applying the blue-green light source with different angles in the shell cavity leads to a similar extent of light diffusion. Moreover, light diffusion was also observed even when applying the blue-green light source from the outside surface of the shell.
(d). A defensive role for bioluminescence
We tested propagation of artificial blue-green light through the aperture of the shell of a range of species of non-luminous marine snails (Trochidae, Neritidae, Columbellidae, Muricidae, Nassariidiae, Cerithiidae, Cerithopsidae) and the very closely related, non-bioluminescent planaxid Planaxis sulcatus Born, 1778. We found no light diffusion or transmittance through the shells of any of these comparative taxa. The light diffusion difference between closely related bioluminescent and non-bioluminescent species suggests that the light-handling capacity of the shell in H. brasiliana has probably evolved in response to the molluscs' bioluminescent capability, although it has yet to be tested in a phylogenetic framework. Furthermore, quantifying the relative transmittance of wavelengths through the shell revealed compelling evidence for co-adaptive characteristics in this system. Indeed, we demonstrated above that the shell has selective transmission for the characteristic wavelength of the bioluminescent light (approx. 502 nm; figure 1), which was the least transmitted compared with the available spectrum of visible white light, being presumably retained for diffusion throughout the shell. Thus, in H. brasiliana, we provide evidence that the shell has probably evolved to support extensive diffusion of the blue-green light signal. We hypothesize that this enhancement of the bioluminescent signal should increase ecological fitness.
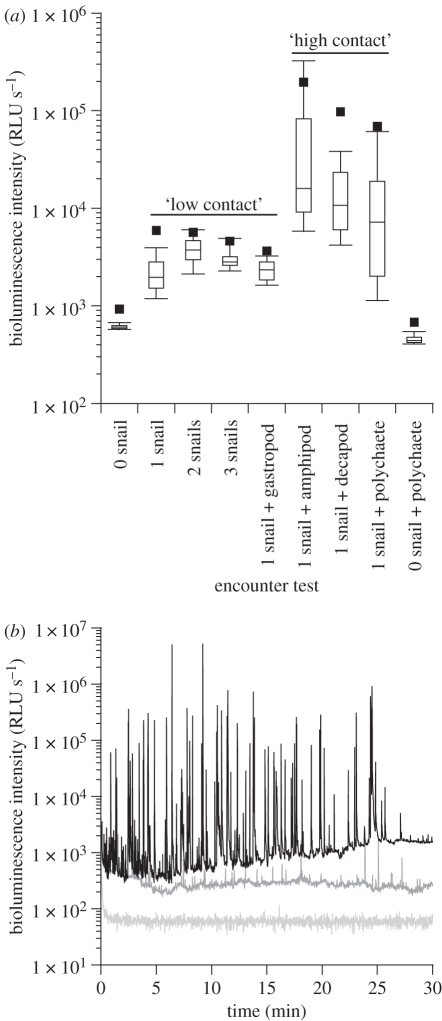
Anecdotal observations on marine snails in the family Planaxidae indicate bioluminescence occurs when the organisms are agitated vigorously in a container [41–43]. Here, we determined if more ecologically relevant conditions (i.e. exposure to other intertidal marine organisms) might elicit the same response. When exposed to high-contact organisms like polychaetes and crustaceans, individuals of H. brasiliana crawling around would rapidly retract into their shells upon every encounter impact, and emerge out again seconds (or sometimes tens of seconds) later. This was similar to when controlled shell taps were applied by the observer on crawling snail individuals. Nonetheless, we were always able to measure luminous activity and identify that H. brasiliana produced a series of short (≤1 s), intense, bioluminescent flashes that were significantly more intense (p < 0.0001) than when exposed to low-contact organisms (figure 4a). The various high-contact organisms tested showed significant differences (p < 0.0026) in their ability to trigger light from H. brasiliana; the amphipod (C. uncinata) triggered the greatest reaction, followed by the decapod (P. macrodactylus) and the polychaete (A. pulchra; figure 4a). The baseline bioluminescent glow was about six times more intense with high-contact organisms than with slow-moving, low-contact organisms, and gradually increased over the time of the encounter, being more than 10× greater by the end of the experiment (figure 4b). There was a dramatic increase in the number and the intensity of light production events upon exposure to high-contact organisms, having up to 100× more flashes (sometimes more than 10 000× more intense) compared to low-contact organisms (figure 4b). The behaviour of H. brasiliana during the low-contact encounter episodes was also different from the high-contact ones; indeed, the snail individuals were then most often extended and crawling around, even when touched by a low-contact organism.
Figure 4.
(a) Box plot representation of the variation of bioluminescence intensity recorded for high- and low-contact organisms and controls. (b) Bioluminescence production profile of H. brasiliana when exposed to one high-contact polychaete (black line) and two low-contact conspecifics (dark grey line); also shown is the no-snail control (light grey line; see electronic supplementary material, figure S3 for expanded flash characteristics). RLU, relative light units.
Encounters with high-contact organisms were analogous to high-energy, acute physical disturbance, which is known to trigger light production in other luminous organisms that use bioluminescence for defence [15,45,49], although mechanical stimulation has rarely been reported to trigger light production in luminous snails [20]. The bioluminescence was not expelled in mucous since removing the snail from the luminometer immediately restored light to background levels (figure 4a, 0 snail + polychaete). During interactions with fast-moving organisms, H. brasiliana repeatedly produced light flashes of high intensity (approx. 2 × 105 to 7 × 104 Relative Light Units; figure 4b). Each flash almost certainly resulted from an encounter impact (or pressure waves caused by a close encounter) with the high-contact organism (which, under dim-light conditions, were observed multiple times per minute). Moreover, the rapid frequency of flash production observed in H. brasiliana only allows a limited time between flashes, which often was less than a few seconds (figure 4b), thus making it unlikely that the snail was repeatedly retracting and emerging from the shell during such brief flash-to-flash intervals.
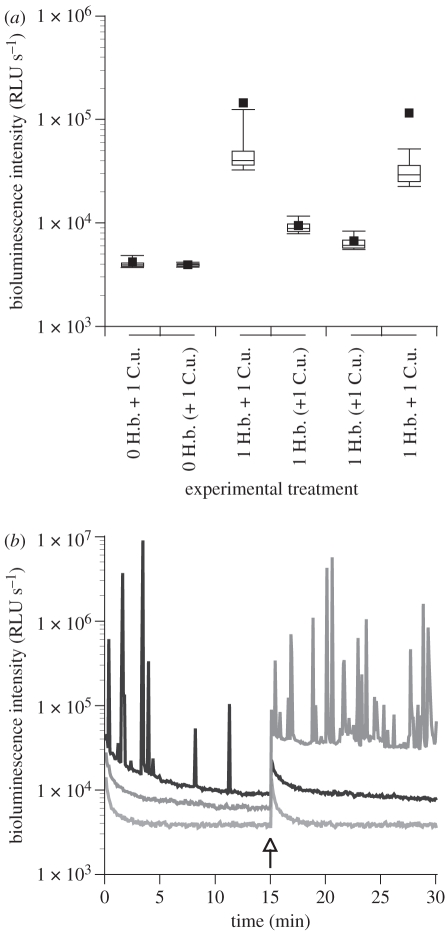
To further elucidate mechanical stimulation of bioluminescence in H. brasiliana, we carried out a second set of experiments with the fast-swimming amphipod C. uncinata and other conspecifics of Hinea. We showed that H. brasiliana produced a significantly greater number of flashes (p < 0.001) when placed together with the amphipod than when separated from it by a plastic divider (figure 5a). This separation technique allowed visual and chemical cues to be exchanged between the compartments of the dish, but precluded physical contact between individuals. Flashes were only observed during the period when the snail and amphipod were together, when mechanical stimulation was possible (figure 5b). This correlation was independent of treatment order. Thus, our results indicated a lack of response to visual and/or chemical cues.
Figure 5.
(a) Box plot representation of the variation of bioluminescence intensity recorded for 0 or 1 snail H. brasiliana (H.b.), combined or separated from the amphipod C. uncinata (C.u.). Brackets indicate when separation with divider was present, while treatments under the same line represent successive paired conditions. (b) Bioluminescence production profile of H. brasiliana when exposed to the amphipod C. uncinata initially separated (dark grey line) or initially combined (black line) using a plastic divider. The light grey line represents background control of the instrument.
In situ, planaxid snails often cluster together in high numbers in moist crevices or under rocks during low tide, giving rise to their common name of ‘clusterwinks’. Although speculative, it is reasonable to assume that a large group of snails that flash when threatened would further deter predation, thus enhancing the ‘flash bulb’ effect that might temporarily blind nocturnal, visual predators [9,50]. It is also possible that the light production acts as an aposematic signal [51–53], but this is less probable considering the gregarious and cryptic behaviour of H. brasiliana. Further experiments are needed to fully characterize the functional aspects of bioluminescence in H. brasiliana.
In comparison, the well-studied terrestrial bioluminescent pulmonate snail Dyakia striata (Gray, 1834) emits light from a glandular organ located on the head, which is expressed as extended flashes/glows up to 6 s long [54]. Light production in D. striata occurs mainly when the foot is extended outside the shell and in response to light stimulation, while being inhibited by mechanical stimulation [55,56]. The function of light emission in D. striata remains uncertain; a communication role has been proposed [57], but is complicated, and hypotheses regarding defence and links to sexual maturity have been variously upheld and dismissed through conflicting results [54,58–60]. Given that a significant period of flashing in D. striata occurs while fully retracted in the shell [54], we suggest that re-investigation of the shell's role in the signal behaviour may clarify some of the conflict recorded to date.
(e). Kinetics of light emission
Hinea brasiliana produces only a small amount of light spontaneously (e.g. figure 5), while it produces intense flashes upon mechanical stimulation (e.g. figure 4). Stimulated light production takes place as a series of intense flashes of short duration of typically less than 0.2–1.0 s, as recorded with a conventional luminometer (0.2 s time resolution; electronic supplementary material, figure S3a). As a result, light production usually appears like the neuro-modulated train of events found in brittlestars [61,62], or like the mechanically driven light production of dinoflagellates [45,63,64]. In some rare cases, and mostly upon mechanical stimulation, the light flashes were dim and so close to one another that the light recording appeared as a long flash, sometimes up to 20 s long. The flash then appears relatively symmetrical, thus showing a similar rise and decay time, both following a single exponential profile (electronic supplementary material, figure S3b).
These two types of flashes could also be mimicked by chemical stimulation. When the neuro-mediator acetylcholine (Ach) was added to the ASW, the light production appeared as a series of short flashes predominantly (electronic supplementary material, S3c), although with an elevated background light production owing to the stimulation protocol that requires drilling (viz. inducing stress) through the shell of the snail. When using KCl to depolarize tissues and trigger massive light production, the bioluminescence then appears as a unimodal flash up to 50 s long, the light increasing first within seconds to a maximal intensity (which combines the time KCl takes to diffuse through the entire animal and the time depolarization takes to reach the luminous organelles in the light-producing cells). After reaching its peak value, the light intensity decreases following a first-order exponential decay, sometimes with a slight shoulder at 20–30 s (electronic supplementary material, figure S3d).
Such kinetics profiles were confirmed when depicting higher-resolution (at 0.010 s) kinetics of spontaneous light production from one single individual, then showing flashes lasting about 150–200 ms. These flashes usually had low-intensity and a jagged profile, and were typically symmetrical (electronic supplementary material, figure S3e). Flashes occurring in response to mechanical stimulation from the amphipod C. uncinata showed a similar profile in being symmetrical, while being more intense, lasting 500–700 ms (or more, depending on the intensity of light production), and most often unimodal (viz. with less of a jagged outline; electronic supplementary material, figure S3f). Kinetics of light production in H. brasiliana thus appears variable, most often showing flashes as opposed to glows (cf. [1]). In any case, the light production appears under nervous control, and probably depends on effector molecules and/or co-factors, which would be reflected by the diversity and jagged features of light flashes observed from H. brasiliana, and in the fact that the flashes often show a symmetrical profile [1,65,66].
(f). Modulation of bioluminescent signals
Bioluminescent organisms can manipulate their light signals through a series of physiological and/or biomechanical mechanisms, such as up- or downregulating the photochemical reaction, reducing the emission area (e.g. [67]), redirecting the signal using reflectors [31] or changing its wavelength using filters [30,68]. Many luminous organisms also show the ability to manipulate their bioluminescent signal to appear spatially larger than the actual light source, which is usually done through the use of a combination of reflectors and transparent biological material that guides the emitted light away from the source [30–33,36,38]. Such mechanisms are often reported from luminous pelagic organisms while, to our knowledge, not yet reported for their benthic counterparts. In the terrestrial realm, some Diptera larvae (glow-worms) show efficient diffusion of bioluminescence down their silk feeding lines [69,70].
In H. brasiliana, the light system differs fundamentally from all other diffusive systems by using a secreted hard non-transparent shell to diffuse light and propagate the luminous signal outward. Protective mechanisms in H. brasiliana thus appear to be threefold: the species possesses a shell for physical protection, shows cryptic behaviour to avoid other organisms, and has the capacity to produce intense, repeated light flashes in response to disturbance. Such efficient light transmittance and wavelength-specific diffusion have not been reported in other invertebrates, and are intriguing given the robust, opaque and glossy shell material. Other naturally occurring diffusers may exist, and we anticipate this study will stimulate re-examination of the potential role that hard structures may play in bioluminescent systems.
(g). General implications
The pattern of bioluminescence described here for H. brasiliana matches key elements of light production previously reported for the closely related species of Planaxis, and might indicate a single origin of bioluminescence in the Planaxidae family. Furthermore, this study provides evidence that the bioluminescence in H. brasiliana is likely to act as a deterrent visual signal against predation, which would be optimized by spatial amplification of a light signal via extensive diffusion through the shell. Both transmission and diffusion through the calcified shell material are greater than that of a comparable commercial diffuser. The selective non-transmission of wavelengths corresponding to the snail's bioluminescence seems to suggest coevolution between light production and biomineralization of the hard calcium carbonate shell. Consequently, understanding how the crystalline and protein structure of the shell allows such properties may prove a valuable and inspiring model for future biophotonic applications.
Acknowledgements
We thank Dr Michael Latz (SIO) for providing access to laboratory space and facilities. We are also grateful to Gregory Rouse, Eric Allen, Karen Osborn, Richard Norris and anonymous reviewers for comments that greatly improved the manuscript. We also thank Dr Rajesh Naik (AFRL) for constructive discussion on experimental design. Research supported by AFOSR FA9550-07-1-0027 (D.D.D.), AFOSR BioOptics MURI FA 9550-09-1-0669 (D.D.D., N.G.W.) and the Mark Mitchell Foundation (N.G.W.).
References
- 1.Hastings W. J., Morin J. G. 1991. Bioluminescence. In Comparative animal physiology. Volume 1: neural and integrative animal physiology (ed. Prosser C. L.), pp. 131–170 New York, NY: John Wiley & Sons [Google Scholar]
- 2.Herring P. J. 1990. Bioluminescent communication in the sea. In Light and life in the sea (eds Herring P. J., Campbell A. K., Whitfield M., Maddock L.), pp. 245–264 Cambridge, UK: University Press [Google Scholar]
- 3.Herring P. J. 1978. Bioluminescence in action. London, UK: Academic Press [Google Scholar]
- 4.Haddock S. H. D., Moline M. A., Case J. F. 2009. Bioluminescence in the Sea. Annu. Rev. Mar. Sci. 2, 293–343 10.1146/annurev-marine-120308-081028 (doi:10.1146/annurev-marine-120308-081028) [DOI] [PubMed] [Google Scholar]
- 5.Herring P. J. 2000. Species abundance, sexual encounter and bioluminescent signalling in the deep sea. Phil. Trans. R. Soc. Lond. B 355, 1273–1276 10.1098/rstb.2000.0682 (doi:10.1098/rstb.2000.0682) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herring P. J. 2007. Sex with the lights on? A review of bioluminescent sexual dimorphism in the sea. J. Mar. Biol. Assoc. UK 87, 829–842 10.1017/S0025315407056433 (doi:10.1017/S0025315407056433) [DOI] [Google Scholar]
- 7.Rivers T. J., Morin J. G. 2008. Complex sexual courtship displays by luminescent male marine ostracods. J. Exp. Biol. 211, 2252–2262 10.1242/jeb.011130 (doi:10.1242/jeb.011130) [DOI] [PubMed] [Google Scholar]
- 8.Herring P. J., Morin J. G. 1978. Bioluminescence in fishes. In Bioluminescence in action (ed. Herring P. J.), pp. 287–329 London, UK: Academic Press [Google Scholar]
- 9.Morin J. G. 1983. Coastal bioluminescence: patterns and functions. Bull. Mar. Sci. 33, 787–817 [Google Scholar]
- 10.Morin J. G., Harrington A., Nealson K., Krieger N., Baldwin T. O., Hastings J. W. 1975. Light for all reasons—versatility in behavioral repertoire of flashlight fish. Science 190, 74–76 [Google Scholar]
- 11.Clarke W. D. 1963. Function of bioluminescence in mesopelagic organisms. Nature 198, 1244–1246 10.1038/1981244a0 (doi:10.1038/1981244a0) [DOI] [Google Scholar]
- 12.Hastings J. W. 1971. Light to hide by—ventral luminescence to camouflage the silhouette. Science 173, 1016–1017 10.1126/science.173.4001.1016 (doi:10.1126/science.173.4001.1016) [DOI] [PubMed] [Google Scholar]
- 13.Lawry J. V. 1974. Lantern fish compare downwelling light and bioluminescence. Nature 247, 155–157 10.1038/247155a0 (doi:10.1038/247155a0) [DOI] [Google Scholar]
- 14.Young R. E., Roper C. F. E. 1976. Bioluminescent countershading in midwater animals: evidence from living squid. Science 191, 1046–1048 10.1126/science.1251214 (doi:10.1126/science.1251214) [DOI] [PubMed] [Google Scholar]
- 15.Deheyn D., Mallefet J., Jangoux M. 2000. Expression of bioluminescence in Amphipholis squamata (Ophiuroidea: Echinodermata) in presence of various organisms: a laboratory study. J. Mar. Biol. Assoc. UK 80, 179–180 10.1017/S0025315499001733 (doi:10.1017/S0025315499001733) [DOI] [Google Scholar]
- 16.Fristrup K. M., Harbison G. R. 2002. How do sperm whales catch squids? Mar. Mamm. Sci. 18, 42–54 10.1111/j.1748-7692.2002.tb01017.x (doi:10.1111/j.1748-7692.2002.tb01017.x) [DOI] [Google Scholar]
- 17.Grober M. 1988. Brittle-star bioluminescence functions as an aposematic signal to deter crustacean predators. Anim. Behav. 36, 493–501 10.1016/S0003-3472(88)80020-4 (doi:10.1016/S0003-3472(88)80020-4) [DOI] [Google Scholar]
- 18.Widder E. A. 1998. A predatory use of counterillumination by the squaloid shark, Isistius brasiliensis. Environ. Biol. Fish. 53, 267–273 10.1023/A:1007498915860 (doi:10.1023/A:1007498915860) [DOI] [Google Scholar]
- 19.Hastings J. W. 1983. Biological diversity, chemical mechanisms, and the evolutionary origins of bioluminescent systems. J. Mol. Evol. 19, 309–321 10.1007/BF02101634 (doi:10.1007/BF02101634) [DOI] [PubMed] [Google Scholar]
- 20.Herring P. J. 1988. Luminescent organs. In The Mollusca: form and function, vol. 11 (eds Trueman E. R., Clarke M. R.), pp. 449–489 New York, NY: Academic Press [Google Scholar]
- 21.Johnsen S., Balser E. J., Fisher E. C., Widder E. A. 1999. Bioluminescence in the deep-sea cirrate octopod Stauroteuthis syrtensis Verrill (Mollusca: Cephalopoda). Ecol. Evol. 197, 26–39 [DOI] [PubMed] [Google Scholar]
- 22.Johnsen S., Balser E. J., Widder E. A. 1999. Light-emitting suckers in an octopus. Nature 398, 113. 10.1038/18131 (doi:10.1038/18131) [DOI] [Google Scholar]
- 23.Jones B. W., Nishiguchi M. K. 2004. Counterillumination in the Hawaiian bobtail squid, Euprymna scolopes Berry (Mollusca: Cephalopoda). Mar. Biol. 144, 1151–1155 10.1007/s00227-003-1285-3 (doi:10.1007/s00227-003-1285-3) [DOI] [Google Scholar]
- 24.Kubodera T., Koyama Y., Mori K. 2007. Observations of wild hunting behaviour and bioluminescence of a large deep-sea, eight-armed squid, Taningia danae. Proc. R. Soc. B 274, 1029–1034 10.1098/rspb.2006.0236 (doi:10.1098/rspb.2006.0236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuji F. I., Leisman G. B. 1981. K+/ Na+-triggered bioluminescence in the oceanic squid Symplectoteuthis oualaniensis. Proc. Natl Acad. Sci. USA 78, 6719–6723 10.1073/pnas.78.11.6719 (doi:10.1073/pnas.78.11.6719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anctil M. 1977. Development of bioluminescence and photophores in the midshipman fish, Porichthys notatus. J. Morphol. 151, 363–395 10.1002/jmor.1051510305 (doi:10.1002/jmor.1051510305) [DOI] [PubMed] [Google Scholar]
- 27.Lohrmann K. B. 2008. Subcutaneous photophores in the jumbo squid Dosidicus gigas (d'Orbigny, 1835) (Cephalopoda: Ommastrephidae). Rev. Biol. Mar. Oceanogr. 43, 275–284 10.4067/S0718-19572008000200006 (doi:10.4067/S0718-19572008000200006) [DOI] [Google Scholar]
- 28.Arnold J. M., Young R. E., King M. V. 1974. Ultrastructure of a cephalopod photophore. II. Iridophores as reflectors and transmitters. Biol. Bull. 147, 522–534 10.2307/1540737 (doi:10.2307/1540737) [DOI] [PubMed] [Google Scholar]
- 29.Butcher S., Dilly P. N., Herring P. J. 1982. The comparative morphology of the photophores of the squid Pyroteuthis margaritifera (Cephalopoda, Enoploteuthidae). J. Zool. 196, 133–150 10.1111/j.1469-7998.1982.tb03497.x (doi:10.1111/j.1469-7998.1982.tb03497.x) [DOI] [Google Scholar]
- 30.Denton E. J., Herring P. J., Widder E. A., Latz M. I., Case J. F. 1985. The roles of filters in the photophores of oceanic animals and their relation to vision in the oceanic environment. Proc. R. Soc. Lond. B 225, 63–97 10.1098/rspb.1985.0051 (doi:10.1098/rspb.1985.0051) [DOI] [Google Scholar]
- 31.Herring P. J. 2000. Bioluminescent signals and the role of reflectors (Review). J. Opt. A Pure Appl. Opt. 2, R29–R38 10.1088/1464-4258/2/6/202 (doi:10.1088/1464-4258/2/6/202) [DOI] [Google Scholar]
- 32.Herring P. J., Dilly P. N., Cope C. 1994. The bioluminescent organs of the deep-sea cephalopod Vampyroteuthis infernalis (Cephalopoda, Vampyromorpha). J. Zool. 233, 45–55 10.1111/j.1469-7998.1994.tb05261.x (doi:10.1111/j.1469-7998.1994.tb05261.x) [DOI] [Google Scholar]
- 33.McFall-Ngai M., Montgomery M. K. 1990. The anatomy and morphology of the adult bacterial light organ of Euprymna scolopes Berry (Cephalopoda, Sepiolidae). Biol. Bull. 179, 332–339 10.2307/1542325 (doi:10.2307/1542325) [DOI] [PubMed] [Google Scholar]
- 34.Herring P. J., Dilly P. N., Cope C. 1985. The photophore morphology of Selenoteuthis scintillans Voss and other Lycoteuthids (Cephalopoda, Lycoteuthidae). J. Zool. 206, 567–589 10.1111/j.1469-7998.1985.tb03559.x (doi:10.1111/j.1469-7998.1985.tb03559.x) [DOI] [Google Scholar]
- 35.Bertelsen E., Munk O. 1964. Rectal light organs in the argentinoid fishes Opisthoproctus and Winteria. Dana Rep. 62, 1–17 [Google Scholar]
- 36.McFall-Ngai M. J. 1983. Adaptations for reflection of bioluminescent light in the gas bladder of Leiognathus equulus (Perciformes, Leiognathidae). J. Exp. Zool. 227, 23–33 10.1002/jez.1402270105 (doi:10.1002/jez.1402270105) [DOI] [PubMed] [Google Scholar]
- 37.Munk O., Bertelsen E. 1980. On the esca light organ and its associated light-guiding structures in the deep-sea anglerfish Chaenophryne draco (Pisces, Ceratioidei). Vidensk. Meddr. Dansk. Naturh. Foren. 142, 103–129 [Google Scholar]
- 38.Munk O., Hansen K., Herring P. J. 1998. On the development and structure of the escal light organ of some melanocetid deep sea anglerfishes (Pisces: Ceratioidei). J. Mar. Biol. Assoc. UK 78, 1321–1335 [Google Scholar]
- 39.Kawahara M., Gleadall I. G., Tsukahara Y. 1998. A note on the fibre-optic light-guides in the eye photophores of Watasenia scintillans. S. Afr. J. Mar. Sci. 20, 123–127 10.2989/025776198784126250 (doi:10.2989/025776198784126250) [DOI] [Google Scholar]
- 40.Harvey E. N. 1952. Bioluminescence. New York, NY: Academic Press [Google Scholar]
- 41.Haneda Y. 1958. Studies on luminescence in marine snails. Pac. Sci. 12, 152–156 [Google Scholar]
- 42.Ponder W. F. 1988. Bioluminescence in Hinea braziliana (Lamarck) (Gastropoda, Planaxidae). J. Molluscan Stud. 54, 361–361 [Google Scholar]
- 43.Houbrick R. S. 1987. Anatomy, reproductive biology, and phylogeny of the Planaxidae (Cerithiacea: Prosobranchia). Smithson. Contrib. Zool. 445, 1–57 [Google Scholar]
- 44.Shimomura O. 2006. Bioluminescence: chemical principles and methods. Singapore: World Scientific [Google Scholar]
- 45.Latz M. I., Lee A. O. 1995. Spontaneous and stimulated bioluminescence of the dinoflagellate Ceratocorys horrida (Peridiniales). J. Phycol. 31, 120–132 10.1111/j.1529-8817.1995.tb03766.x (doi:10.1111/j.1529-8817.1995.tb03766.x) [DOI] [Google Scholar]
- 46.Maldonado E. M., Latz M. I. 2007. Shear-stress dependence of dinoflagellate bioluminescence. Biol. Bull. 212, 242–249 [DOI] [PubMed] [Google Scholar]
- 47.Zar J. H. 1996. Biostatistical analysis. Upper Saddle River, NJ: Prentice-Hall [Google Scholar]
- 48.Widder E. A., Latz M. I., Herring P. J., Case J. F. 1984. Far red bioluminescence from two deep-sea fishes. Science 225, 512–514 10.1126/science.225.4661.512 (doi:10.1126/science.225.4661.512) [DOI] [PubMed] [Google Scholar]
- 49.Zorner S. A., Fischer A. 2007. The spatial pattern of bioluminescent flashes in the polychaete Eusyllis blomstrandi (Annelida). Helgoland Mar. Res. 61, 55–66 10.1007/s10152-006-0053-4 (doi:10.1007/s10152-006-0053-4) [DOI] [Google Scholar]
- 50.Grober M. 1988. Responses of tropical reef fauna to brittle-star luminescence (Echinodermata: Ophiuroidea). J. Exp. Mar. Biol. Ecol. 115, 157–168 10.1016/0022-0981(88)90100-1 (doi:10.1016/0022-0981(88)90100-1) [DOI] [Google Scholar]
- 51.Grober M. S. 1989. Aposematism and bioluminescence. Anim. Behav. 37, 341–343 10.1016/0003-3472(89)90127-9 (doi:10.1016/0003-3472(89)90127-9) [DOI] [Google Scholar]
- 52.Grober M. S. 1990. Luminescent flash avoidance in the nocturnal crab Portunus xantusii. 1. The effects of luminescence and mechanical stimulation on heart rate. J. Exp. Biol. 148, 415–426 [Google Scholar]
- 53.Grober M. S. 1990. Luminescent flash avoidance in the nocturnal crab Portunus xantusii. 2. Cardiac and visual responses to variations in simulated luminescent flashes. J. Exp. Biol. 148, 427–448 [Google Scholar]
- 54.Copeland J., Daston M. M. 1993. Adult and juvenile flashes in the terrestrial snail Dyakia striata. Malacologia 35, 1–7 [Google Scholar]
- 55.Copeland J. 1988. Optic nerve responses to light stimulation in the bioluminescent terrestrial snail, Dyakia (Quantula) striata. Comp. Biochem. Physiol. 89A, 391–400 10.1016/0300-9629(88)91046-8 (doi:10.1016/0300-9629(88)91046-8) [DOI] [Google Scholar]
- 56.Isobe M., Uyakul D., Goto T., Counsilman J. J. 1988. Dyakia bioluminescence. 1. Bioluminescence and fluorescence spectra of the land snail, Dyakia striata. J. Biolum. Chemilum. 2, 73–79 10.1002/bio.1170020204 (doi:10.1002/bio.1170020204) [DOI] [PubMed] [Google Scholar]
- 57.Copeland J., Daston M. M. 1989. Bioluminescence in the terrestrial snail Dyakia (Quantula) striata. Malacologia 30, 317–324 [Google Scholar]
- 58.Counsilman J. J., Loh D., Chan S. Y., Tan W. H., Copeland J., Maneri M. 1987. Factors affecting the rate of flashing and loss of luminescence in an Asian land snail, Dyakia striata. Veliger 29, 394–399 [Google Scholar]
- 59.Counsilman J. J., Ong P. P. 1988. Responses of the luminescent land snail Dyakia (Quantula) striata to natural and artificial lights. J. Ethol. 6, 1–8 10.1007/BF02348856 (doi:10.1007/BF02348856) [DOI] [Google Scholar]
- 60.Daston M. M., Copeland J. 1993. The luminescent organ and sexual maturity in Dyakia striata. Malacologia 35, 9–19 [Google Scholar]
- 61.De Bremaeker N., Mallefet J., Baguet F. 1996. Luminescence control in the brittlestar Amphipholis squamata: effect of cholinergic drugs. Comp. Biochem. Physiol. C 115, 75–82 10.1016/S0742-8413(96)00059-X (doi:10.1016/S0742-8413(96)00059-X) [DOI] [Google Scholar]
- 62.Brehm P. 1977. Electrophysiology and luminescence of an ophiuroid radial nerve. J. Exp. Biol. 71, 213–227 [DOI] [PubMed] [Google Scholar]
- 63.Latz M. I., Nauen J. C., Rohr J. 2004. Bioluminescence response of four species of dinoflagellates to fully developed pipe flow. 26, 1529–1546 10.1093/plankt/fbh141 (doi:10.1093/plankt/fbh141) [DOI] [Google Scholar]
- 64.Widder E. a., Case J. F. 1981. Two flash forms in the bioluminescent dinoflagellate, Pyrocystis fusiformis. 143, 43–52 [Google Scholar]
- 65.Hastings W. J. 1995. Bioluminescence. In Cell physiology, vol. 31 (ed. Sperelakis N.), pp. 665–681 New York, NY: Academic Press [Google Scholar]
- 66.Wilson T., Hastings J. W. 1998. Bioluminescence. Ann. Rev. Cell Dev. Biol. 14, 197–230 10.1146/annurev.cellbio.14.1.197 (doi:10.1146/annurev.cellbio.14.1.197) [DOI] [PubMed] [Google Scholar]
- 67.Herring P. J. 1977. Bioluminescence of marine organisms. Nature 267, 788–793 10.1038/267788a0 (doi:10.1038/267788a0) [DOI] [Google Scholar]
- 68.Herring P. J. 1983. The spectral characteristics of luminous marine organisms. Proc. R. Soc. Lond. B 220, 183–217 10.1098/rspb.1983.0095 (doi:10.1098/rspb.1983.0095) [DOI] [Google Scholar]
- 69.Broadley R. A., Stringer I. A. N. 2001. Prey attraction by larvae of the New Zealand glowworm, Arachnocampa luminosa (Diptera: Mycetophilidae). Invertebr. Biol. 120, 170–177 10.1111/j.1744-7410.2001.tb00121.x (doi:10.1111/j.1744-7410.2001.tb00121.x) [DOI] [Google Scholar]
- 70.Sivinski J. M. 1998. Phototropism, bioluminescence, and the Diptera. Fla Entomol. 81, 282–292 [Google Scholar]