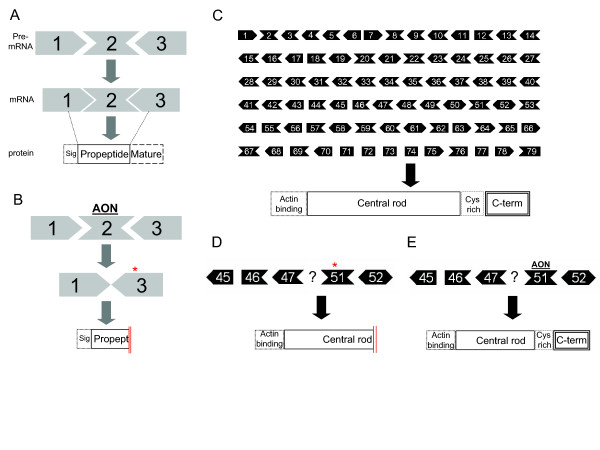
Figure 1.
Schematic overview of myostatin and dystrophin exon skipping. The myostatin gene (MSTN) consists of three exons, whereas the protein consists of three domains: signaling (sig.), propeptide and mature domains. The position of each exon relative to each domain is denoted by broken lines (A). Antisense oligonucleotides (AON) targeting exon 2 will hybridize and hide the exon from the splicing machinery, resulting in skipping exon 2 upon mRNA splicing. The removal of exon 2 will disrupt the open reading frame (ORF; *) and the protein will lack part of the propeptide and the entire mature domain (B). Dystrophin gene (DMD) consists of 79 exons, whereas the proteins consist of actin binding-, central rod, cysteine rich- and C-terminal domains (C). One of the examples of DMD is deletion in exon 48-50 which disrupted the reading frame and introduces premature stop codon (*). Due to this mutation, part of the central rod and the entire Cys-rich and C-terminal domains are missing (D). In the therapy (currently in clinical trials), AON is directed towards exon 51. With the similar principle, exon 51 will be skipped upon mRNA splicing which in turn restores the ORF. This internally-deleted DMD will be translated as dystrophin with shorter central rod domain. However, since the essential C-terminal domain is retained, the protein is partially functional (E).