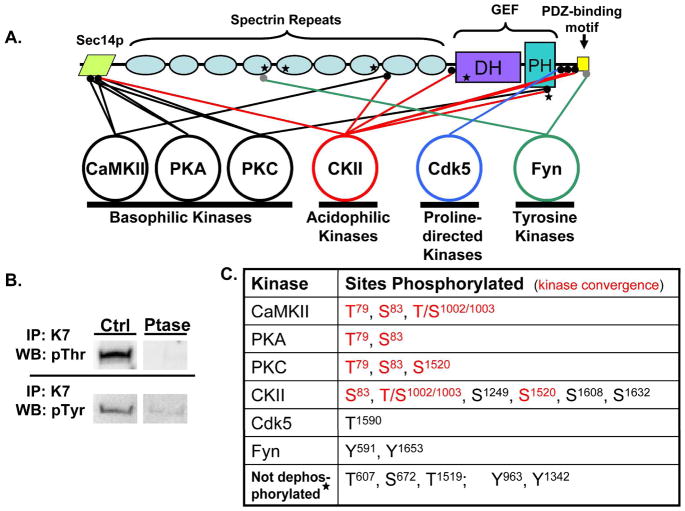
Figure 1.
A. Purified myc-Kal7 is phosphorylated by recombinant protein kinases of different classes. Myc-Kal7 purified from transiently transfected pEAK Rapid cells was dephosphorylated as described in Methods. Recombinant protein kinases representative of four different kinase classes were then incubated with myc-Kal7 and 200 μM ATP under optimal conditions for each enzyme. LC-MS/MS analysis of tryptic peptides from each sample revealed multiple phosphorylation sites for each of the kinases except Cdk5, which had previously been shown to phosphorylate a single site in Kal727. Sites that were not dephosphorylated by phosphatase treatment are indicated by a star; Thr607 and Ser672 are consensus CKII sites (NetPhos 1.0). B. Immunoprecipitated myc-Kal7 was treated with Lambda phosphatase or CIP and then visualized with antibody specific for P-Thr or P-Tyr, respectively. Equal loading of myc-Kal7 was determined by the presence of a single Coomassie-stained band at the correct molecular weight that was not in an immunoprecipitate from non-transfected cells (not shown). C. Sites phosphorylated by each kinase are listed. Red lettering represents sites where multiple kinases converged on a single residue. It was not possible to distinguish phosphorylation of Thr1002 from phosphorylation of Ser1003.