Abstract
Background
Chronic medical conditions such as opioid dependence require evidence-based treatment recommendations. However, pregnant women are underrepresented in clinical trials. We describe the first within-subject comparison of maternal and neonatal outcomes for methadone vs. buprenorphine exposed pregnancies. Though methadone is the established treatment of pregnant opioid dependent women, recent investigations have shown a trend for a milder neonatal abstinence syndrome (NAS) under buprenorphine. However, it is not only the choice of maintenance medication that determines the occurrence of NAS, other factors such as maternal metabolism, illicit substance abuse and nicotine consumption also influence its severity and duration and represent confounding factors in the assessment of randomized clinical trials.
Case series description
Three women who were part of the European cohort of a randomized, double-blind multicenter trial with a contingency management tool [the Maternal Opioid Treatment: Human Experimental Research (MOTHER) study], each had two consecutive pregnancies and were maintained on either methadone or buprenorphine for their first and then the respective opposite, still-blinded medication for their second pregnancy. Birth measurements, the total neonatal abstinence score, the total amounts of medication used to treat NAS and the days of NAS treatment duration were assessed.
Results
Both medications were effective and safe in reducing illicit opioid relapse and avoiding preterm labour. Methadone maintenance yielded to a significantly higher neonatal birth weight. Data patterns suggest that buprenorphine-exposure was associated with lower neonatal abstinence syndrome (NAS) scores. Findings from this unique case series are consistent with earlier reports using between-group analyses.
Conclusions
Buprenorphine has the potential to become an established treatment alternative to methadone for pregnant opioid dependent women. Under special consideration of ethical boundaries, psychopharmacological treatment during pregnancy must be addressed as an integral part of clinical research projects in order to optimize treatment for women and neonates.
Keywords: opioid dependence, methadone, buprenorphine, pregnancy, neonatal abstinence syndrome
Background
The involvement of pregnant women in clinical research trials is a complex issue, especially when evaluating the safety and efficacy of medical drugs [1]. The concept of the fetus as a patient, who cannot be asked to sign informed consent [2], [3], neonatal safety as a main determinant of study design, and the pregnant patient’s obligation towards the fetal patient [4] represent challenging ethical issues. A history of paternalistic and exclusionary policies toward women (e.g., only in 1993 did the US Food and Drug Administration lift the ban on pregnant women from clinical trial participation[5]) has resulted in a general lack of randomized controlled trials (RCTs) with pregnant participants for medications used to treat even such common conditions as asthma, depression and epilepsy[1]. Similar considerations apply to the high risk population of opioid dependent women during pregnancy, a major public health issue in need of evidence-based guidelines in order to optimize treatment approaches.
Although opioid agonist maintenance treatment is indicated as first line therapy for opioid dependent pregnant women, [6] only two pilot RCTs have provided maternal and neonatal outcome data comparing the safety and efficacy of methadone and buprenorphine[7,8] – however, still a unique situation, as so far prospective double-blind double-dummy designed RCTs involving pregnant women have rarely been available even for common medical conditions such as hypertension or asthma. Consistent with prior investigations, [9, 10] these two RCTs involving pregnant opioid dependent women, supported the safety and efficacy of both buprenorphine and methadone and one study[7] demonstrated significant reductions in neonatal hospitalization duration with prenatal-buprenorphine exposure. The duration and severity of NAS is influenced by multiple factors which are difficult to control for in clinical trials, such as nicotine [11], and other drug use [8], medication dose, and maternal medication metabolism.
On the basis of these pilot studies, the largest research effort in this field has recently been completed [15]: The Maternal Opioid Treatment: Human Experimental Research (MOTHER) project a multi-centre, randomized, double-blind, double-dummy, clinical trial, involving eight sites in North America and Europe, [12, 13, 14]designed to evaluate maternal and neonatal safety and efficacy of methadone and buprenorphine. We examined both maternal and fetal outcomes of three women who had two consecutive pregnancies while enrolled in the MOTHER study. All three women were maintained on either buprenorphine or methadone for their first pregnancy, (medication remained blinded to participants and all staff except the pharmacist throughout both pregnancies), and then the respective opposite medication for their second pregnancy. Outcome parameters were maternal and neonatal safety and efficacy, severity and duration of the neonatal abstinence syndrome, the amount of NAS medication administered and birth outcome parameters such as weight, length and head circumference.
The ethics committee at the Medical University of Vienna approved the MOTHER trial (INB number 451/1998). All participants signed informed consent for both pregnancies.
Case descriptions
Procedures
Enrolment and randomization procedures – inpatient setting
Participants completed an assessment battery including the structured clinical interview for DSM-IV Axis I disorders (SCID) interview [16] and the Addiction Severity Index (ASI) [17] to determine eligibility. For their first pregnancy, participants were randomized to either methadone or buprenorphine based on a permutation procedure at the Center of Substance Abuse Research (CESAR, University of Maryland). CESAR staff notified the Vienna Site’s pharmacy of the participant’s medication group. In both studies participants received supervised medication (matched with placebo tablets/solution) in a double-blind, double-dummy fashion.
Before transition to the double-blind study medication, participants received rapid release morphine as a wash-out medication for three days and were hospitalized for induction onto the study medication (methadone or buprenorphine).
Study procedures – outpatient setting
All subjects visited the Vienna addiction clinic daily and received their double-blind study medication once daily in the morning. Supervised urine specimens and alcohol breathalyzer tests were collected three times a week. Nicotine consumption was assessed by oral self-report measures. All participants were provided with obstetric care by gynaecologists of the Vienna University hospital which included regular fetal monitoring (cardio tocograph CTGs) and ultrasound scans. All participants were provided with psychiatric treatment and were also seen by a social worker on a regular basis. They also had the opportunity to attend an expert-guided peer group once a week on a voluntary basis.
Flexible dosing schedule
Treatment was initiated at the equivalent of the prior opioid maintenance dose (slow release oral morphine (SROM)/methadone/buprenorphine) each woman had been receiving, with a minimum dose of 2 mg buprenorphine or 10 mg methadone. Medication levels were blindly-adjusted as required with a maximum level of 32 mg buprenorphine/140 mg methadone, respectively.
Contingency management procedures
Throughout the prenatal period, participants were eligible to receive monetary vouchers in exchange for drug negative urine toxicology screens for opiates (excluding methadone and buprenorphine), cocaine, amphetamines and benzodiazepines. [7]
Neonatal Abstinence Syndrome (NAS) assessment
NAS was assessed by trained nurses (blind for intra-uterine medication exposure) every four hours using a validated [18] modified version of the Finnegan scale [19], comprised of 19 opioid-withdrawal signs (heightened Moro reflex, tremors, increased muscle tone, high pitch crying, poor sucking etc.) to determine the need of treatment. [7] All neonates received inpatient care for a minimum of 10 days. Infants who required NAS treatment for longer than 10 days remained in the hospital until weaning from NAS medication was complete. The daily peak NAS scores were summed to provide a total NAS score and the amount of medication administered over the entire course of NAS treatment was summed and these average sums were compared between medication groups.
NAS treatment protocol
NAS was treated with morphine drops (0.4 mg/ml or 0.05% Morphine HCl) dosed according to the total NAS score. Treatment was initiated when the NAS score was equal to or greater than 9, with morphine doses (in parentheses) selected according to the NAS score as follows: 0–8 (0.04 mg/dose), 9–12 (0.04 mg/dose), 13–16 (0.08 mg/dose), 17–20 (0.12 mg/dose), 21–24 (0.16 mg/dose), ≥ 25 (0.20 mg/dose) [7, 15].
Statistics
Due to the number of subjects (n=3 mothers/6 neonates) each mother is outlined using the format of a case description. Additional statistical analysis was conducted using SPSS 16.0 for Windows. Baseline and demographic data were assessed using (i) frequencies for categorical variables and (ii) means and standard deviations for continuous variables. Chi-square tests were performed to assess relationships between categorical variables. Maternal background variables were calculated applying dependent samples t-tests, while neonatal outcome parameters were compared using independent samples t-tests to test for differences between the opioid medications. For NAS evaluation a 16 day data monitoring was regarded relevant as the last neonate was off medication by day 15. Analyses for NAS score and medication were done by the General Linear Model (GLM) for repeated measures with maternal medication (methadone vs. buprenorphine) and treatment days (16 levels) as within-subject variables. F-tests were carried out for Medication and Days(time) effects as for the interaction effect (IA) Days * Medication. Following the characteristics of the time course of the adopted Finnegan scores and the morphine treatment polynomial contrasts were used for the main effect of time and the interaction Days * Medication was tested using the linear and the quadratic component. Inferential statistics using a significance level of 0.10 was accompanied by assessment of effect sizes. Effect sizes used were twofold, Cohen′s d for comparisons of means using t-tests and partial eta2 for comparisons of means using GLM for repeated measures. Cohen′s d >0.50 and eta2 >0.25 were regarded as moderate, Cohen′s d >0.80 and eta2 >0.40 as large effects.
Results
Case 1
Mother 1 was a 25 year-old unmarried woman who was enrolled for her first pregnancy and transitioned from 300mg slow release oral morphine to 50mg methadone. She had 18.3% positive urine screens, co-occurring nicotine consumption of 15 cigarettes in 24h, and received 90 mg methadone at delivery. She required study medication dose increases during both pregnancies. Her first baby was among the most severely affected by NAS, it had the longest duration of NAS symptoms (12 days) of the six babies and received 3.15 grams total of NAS morphine medication. With a birth weight of 3510g it was the heaviest of the six neonates. This mother became pregnant again less than a year after her first pregnancy, and was again enrolled to the MOTHER trial. She was transitioned from 50mg methadone to 8mg buprenorphine with a final dose of 16mg at delivery. Her second baby with a birthweight of 3110g had very mild symptoms of NAS that did not require any medication. (see table 1).
Table 1.
Individual baseline characteristics, study parameters, birth outcome
| Mother 1 | Mother 2 | Mother 3 | ||||
|---|---|---|---|---|---|---|
| Baby | Baby 1 | Baby 2 | Baby 1 | Baby 2 | Baby 1 | Baby 2 |
|
| ||||||
| Medication exposure | Met | Bup | Met | Bup | Bup | Met |
|
| ||||||
| Gender | male | female | female | male | male | female |
| Mothers age delivery (years) | 25 | 26 | 24 | 25 | 34 | 35 |
| Est. gestational age at study entry (weeks) | 14 | 28 | 15 | 23 | 15 | 21 |
| Parity | 1 | 2 | 2 | 3 | 5 | 6 |
| Number of study days | 192 | 93 | 194 | 136 | 211 | 141 |
| Voucher sum (Euro) | 2201 | 766 | 2227 | 892 | 2310 | 1344 |
| Cigarettes (per 24h) | 15 | 15 | 10 | 20 | 20 | 20 |
| Prior medication | 300 SROM* | 50 Met | 50 SROM* | 40 Met | 8 Bup | 18 Bup |
| Morphine dose at induction (mg) | 250 | 300 | 65 | 180 | 240 | 540 |
| Medication at delivery | 90mg | 16mg | 40mg | 8mg | 16mg | 115mg |
| Medication 10 days post partum | 90mg | 10mg | 40mg | 8mg | 14mg | 100mg |
| Pos. urine screen in % | 18.3 | 7.9 | 15.6 | 21.8 | 11.7 | 11.8 |
| NAS med. (grams total) | 3.15 | 0 | 0.68 | 1.44 | 1.80 | 3.48 |
| NAS med. days treatm. | 12 | 0 | 5 | 6 | 8 | 10 |
| Birth weight (grams) | 3510 | 3110 | 3190 | 2890 | 2680 | 2940 |
| Birth length (cm) | 49 | 50 | 52 | 49 | 52 | 50 |
| Head circumf. (cm) | 35 | 34 | 34 | 33 | 36 | 33 |
| EGA at birth (days) | 268 | 263 | 276 | 264 | 287 | 266 |
SROM = slow release oral morphine
Case 2
Mother 2 was a twenty-four year old woman who was transitioned from a very low dose of 50mg slow release oral morphine to the first study drug methadone, initially 10mg for her first pregnancy. She also required increasing doses throughout both pregnancies and was stabilized on 40mg methadone at her first delivery. Her first baby developed moderate NAS symptoms and had a treatment of five days and only 0.68grams medication which was the lowest amount of medication given. For her second pregnancy, she was transitioned from 40mg methadone to the study drug buprenorphine, initially 6mg under which she had 21.8% positive urine screens. She also doubled her nicotine intake from her first to her second pregnancy. At time of delivery after one dose increase she was stabilized on 8mg buprenorphine. Her second baby received a total of 1.44 grams of NAS medication and was in treatment for six days.
Case 3
Mother 3 was quite different in terms of sociodemographic characteristics compared to the other two mothers as she was pregnant for the fifth time in her life at age 34 and for the first time participated in a study. She was transitioned from 8mg buprenorphine to the first study drug buprenorphine, initially 8mg and requested dose increases reaching a level of 16mg at delivery. She spent 211 days in the study for her first pregnancy which was the longest duration of study participation. Her first baby exhibited strong symptoms of NAS and was treated for eight days receiving a total of 1.8grams of morphine medication. Soon after her baby was born she became pregnant for the sixth time and was enrolled in the trial again. She was transitioned from 18mg buprenorphine to 90mg methadone (second study drug). Because of her many pregnancies she requested a tubal ligation and therefore had an elective caesarian section in EGA week 38. Her second baby required the highest amount of NAS medication of all babies included in this substudy. She was in treatment for ten days with a total of 3.48 grams of NAS medication.
Comparative Data
Maternal Urine Toxicology Screens
The majority of urine toxicology samples collected from mothers maintained on methadone (n=218 specimens) or buprenorphine (n=187) were negative [91.7% (Met) and 88.8% (Bup) samples, respectively] for opioids, cocaine, amphetamines and benzodiazepines. There were no significant differences between the methadone- and buprenorphine-maintained conditions in the percentage of urine analysis results that were positive for any opiates [5.5% (Met) vs. 10.7% (Bup), p=0.578], cocaine [2.3% (Met) vs. 5.3% (Bup), p=0.671], amphetamines [none], benzodiazepines [4.6% (Met) vs. 0 (Bup), p=0.199], or any of these in total [8.3% (Met) vs. 11.2% (Bup), p=0.853]. The discrepancy between the voucher sums attained (about 600 Euros less for the buprenorphine condition) is explained by on average five weeks later study inclusion compared to methadone condition (see table 2).
Table 2.
Comparison between methadone and buprenorphine for maternal variables (upper part) and birth outcome parameters (lower part)
| Methadone | Buprenorphine | p | d | |
|---|---|---|---|---|
| Mothers age at delivery (years) | 28.36 (6.63) | 28.94 (5.09) | 0.597 | 0.10 |
| Estimated gestational age at study entry (weeks) | 16.67 (3.79) | 22.00 (6.56) | 0.463 | 0.99 |
| Parity | 3.00 (2.65) | 3.33 (1.53) | 0.667 | 0.15 |
| Number of study days | 175.67 (30.04) | 146.67 (59.72) | 0.626 | −0.61 |
| Voucher sum ( Euro) | 1924.00 (502.46) | 1322.67 (857.37) | 0.767 | −0.86 |
| Cigarettes smoked (per 24h) pre partum | 16.67 (5.77) | 18.33 (2.89) | 0.742 | 0.36 |
| Morphine dose at induction (mg) | 285.00 (239.43) | 240.00 (60.00) | 0.760 | −0.26 |
| Study medication dosage (EGA 28) | Level 5.33 (4.73) =63.33mg met | 5.00 (2.00) = 10mg bup | 0.860 | −0.09 |
| Study medication dosage delivery | Level 7.00 (3.61) = 80mg met | 6.67 (2.31) = 13.33mg bup | 0.742 | −0.11 |
| Study medication dosage 10 days postpartum | Level 7.00 (3.61) = 80mg met | 5.33 (1.53) = 10.5mg bup | 0.338 | −0.60 |
|
|
|
|
|
|
| Birth weight (g) | 3259.73(285.72) | 2846.93(215.02) | 0.046 | −1.63 |
| Birth length (cm) | 50.33 (1.53) | 50.33 (1.53) | 1.000 | 0 |
| Head circumference (cm) | 34.0 (1.00) | 34.33(1.53) | 0.768 | 0.24 |
| EGA at birth (days) | 270 (5.29) | 271.33(13.58) | 0.882 | 0.13 |
| Duration of NAS treatment (days) | 9.0 (3.6) | 4.7 (4.2) | 0.385 | −1.10 |
Results concerning birth outcome parameters
Effect sizes were low for gestational age, length and head circumference (d<0.25), but were extremely high for the differences in birth weight, indicating that methadone exposed neonates were about 400 grams heavier than these in the buprenorphine condition (see table 2).
Results related to the Neonatal Abstinence Syndrome (NAS)
All six neonates exhibited signs of NAS. Figure 1 displays the course of both the adopted Finnegan scores and the morphine application in the medication exposed neonates. The mean total NAS score (sum of NAS daily peak scores) was 119.7 points (SD=30.7) for the methadone and 86.7 points (SD=28.8) for the buprenorphine condition, respectively. The repeated measures analysis of variance yielded a significant Days effect (F(15,30)=7.104, p<0.001, eta2=0.640), a Medication effect that was close to significance (F(1,2)=5.665, p=0.124, eta2=0.739), but a non-significant Days * Medication interaction (F(15,30)=1.204, p=0.629, eta2=0.174). Testing specifically for significance in terms of a linear or quadratic trend, the quadratic component of the interaction Days * Medication reached levels of significance (F(1,2)=10.542, p=0.083, eta2=0.841). The mean total amount of NAS medication required was 2.5mg (SD=1.6) for methadone-exposed neonates and 1.2mg (SD=1.1) for those exposed to buprenorphine. The Days effect was significant (F(15,30)=4.460, p<0.001, eta2=0.518), while both the Medication effect (F(1,2)=1.163, p=0.394, eta2=0.256) and the interaction Days * Medication (F(15,30)=1.528, p=0.117, eta2=0.284) were not. Contrasts in this case did not show a significant linear (F(1,2)=0.883, p=0.447, eta2=0.306) nor quadratic component (F(1,2)=0.862, p=0.451, eta2=0.301) of the interaction.
Figure 1.
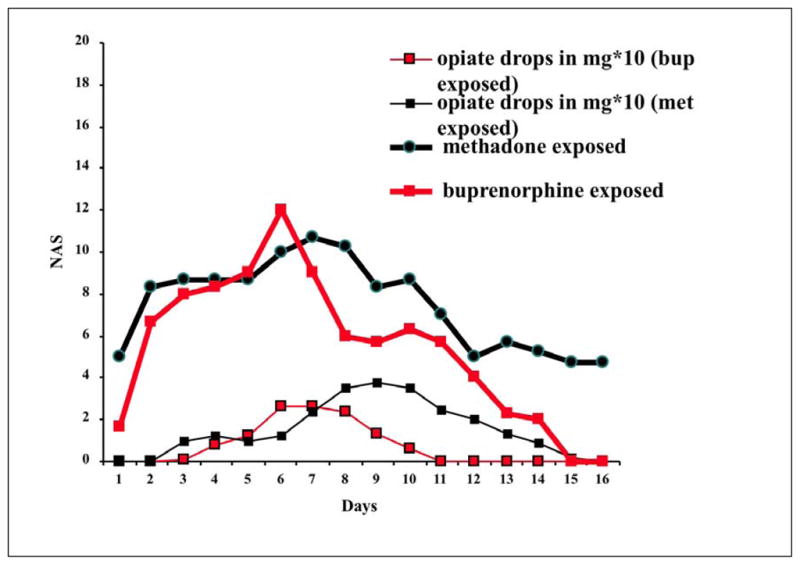
Time course of NAS symptoms over 16 days following birth.
Y-axis shows mean daily adopted Finnegan scores for methadone (---) and buprenorphine (
 ) exposed neonates. Lower curves show neonatal abstinence syndrome treatment with morphine drops in mg*10 for methadone (---) and buprenorphine (
) exposed neonates. Lower curves show neonatal abstinence syndrome treatment with morphine drops in mg*10 for methadone (---) and buprenorphine (
 ) exposed neonates.
) exposed neonates.
X-axis shows days of neonatal abstinence syndrome (NAS) assessment/treatment.
All three methadone exposed neonates required NAS treatment, while only two of the three buprenorphine exposed ones did. Treatment for neonates in the methadone condition started on days 3, 6 and 6, and for the two neonates in the buprenorphine condition on days 5 and 3. The treatment duration lasted 9.0 days for the methadone (SD=3.6) and 4.7 (SD=4.2) for the buprenorphine condition, respectively (p=0.385, d=−1.10). Taking into account birth order as a confounding variable, no significant difference between first and consecutive pregnancies was revealed when viewed independent of substances in terms of NAS score (13.67, SD=43.00, p=0.637, d=−0.39) and medication required (0.31, SD=2.54, p=0.851, d=−0.20).
Discussion
The case series presented is unique in that two different opioid maintenance medications were investigated in opioid-dependent pregnant women using a within-subject design. The analysis of data from three women who had consecutive pregnancies during the study, whilst maintained on double-blind methadone or buprenorphine, provided a unique opportunity to control for factors such as maternal metabolism and personal constitution, which may influence the appearance, severity and duration of NAS.
Both methadone and buprenorphine were effective maintenance medication for all three women in both pregnancies in terms of retaining all three women in treatment for the duration of both pregnancies and in keeping consumption of illicit substances to a minimum. All six neonates were healthy and birth outcome measurements were within a normal range for each baby, regardless of study medication, demonstrating fetal safety of both methadone and buprenorphine.
Compared to previous populations, the cohort evaluated in this study over a long period (average duration of study participation: 23 weeks) was characterized by very low rates of concomitant consumption of illicit substances (approximately 10% of urine toxicology samples were positive), possibly due to the use of daily clinical contacts including supervised dosing and voucher incentives, a form of contingency management shown to improve retention rates especially during pregnancy [20–22]. In order to answer the scientific questions posed in the MOTHER study regarding the safety and efficacy of prenatal exposure to methadone or buprenorphine, a highly rigorous design was required. Such rigid scientific protocols are important on the one hand but limit the ability to generalize from these results to the broader population of opioid-dependent pregnant patients such as those who have concurrent benzodiazepine and/or alcohol abuse or dependence.
Although all three women were heavy smokers, there were no significant differences in smoking behaviour between the two pregnancies. The majority of opioid dependent women are co-dependent on nicotine, [23,24] but few studies have controlled for nicotine dependence. [11] Collectively, these factors suggest that comparisons of neonatal outcomes for methadone and buprenorphine-exposed neonates in this unique cohort may have been less susceptible to several known confounding influences.
Consistent with previous reports, [7,8,15, 25–27] our results revealed a trend in favour of buprenorphine for a milder neonatal abstinence syndrome, lower scores and less days in treatment. Two of three women had milder NAS occurrence altogether under buprenorphine with one buprenorphine exposed neonate not requiring any NAS treatment. Compared to methadone-exposed neonates, those exposed to buprenorphine had fewer NAS symptoms. Differences of NAS treatment doses though were too small to reach statistical significance. This might be explained by the medication dosing protocol used, which followed an escalating regimen that started treatment of neonates only when the predefined cut-off score of the adapted Finnegan scale was surpassed.
Looking at the results for birth outcome measurements, all six neonates were comparable to a non-opioid but nicotine-dependent population. Interestingly, methadone-exposed neonates had a higher birth weight compared to buprenorphine exposed ones, possibly due to the same mechanism responsible for the weight gain commonly observed in methadone-maintained patients. A recent prospective survey of 41 pregnant women found a higher birth weight for the methadone group, approximately 200g above average findings, however, differences in birth weight means were not statistically significant. [28] In fact, the majority of prior studies in the field report the opposite of our finding, all showing a trend for a higher birth weight for buprenorphine exposed neonates. Kakko et al. reported a significantly higher birth weight for buprenorphine exposed neonates [27] while other studies found a similar yet insignificant trend [7, 25] or no differences [8].
Previous authors have questioned whether higher opioid maintenance doses will lead to a milder occurrence of NAS by minimizing illicit substance abuse. [27] In our study, the use of a flexible dosing schedule, with average maximum doses in the upper range (methadone 80 mg, buprenorphine 13 mg), was associated with minimal rates of concomitant consumption. To date, only a few studies reported a correlation between maternal dose and NAS severity; [29,30] other reports have found no such correlation [7,8,25,28,31,32]so that findings, so far, have been inconclusive. A recent systematic review and meta-analysis including 67 studies on methadone dose and neonatal abstinence syndrome concluded that the severity of NAS is not associated with maternal maintenance dose. [33]
These results are in-line with prior research, suggesting that both methadone and buprenorphine are effective and safe in the treatment of opioid dependent pregnant women. Little has been known about whether one or the other might be beneficial to one individual based on biological determinants such as metabolism. Rather than restricting opioid dependent pregnant women to the “gold standard” of methadone for treatment, especially in light of the more favourable NAS occurrence for buprenorphine, each woman should have the choice between buprenorphine or methadone as an effective and safe treatment of opioid dependence during pregnancy. So far neither medication has FDA/EMEA approval for the treatment of pregnant women.
Up to this point, most medication research conducted on pregnant women with chronic medical conditions such as HIV, psychiatric disorders or hypertension has been observational, retrospective, or epidemiological [1, 34]. Furthermore, most trials did not evaluate different classes of medical drugs for the same condition. However, there is a strong need and an ethical justification for further research specifically targeting pregnant women based on existing concepts for the criteria of initiating early-phase investigations and controlled trials, and to evaluate when an experimental intervention should become standard of care [4, 35]. The MOTHER trial may serve as a role model for randomized controlled trials involving pregnant women in the future.
Acknowledgments
We would especially like to thank the nursing staff of the Vienna Addiction Clinic for their dedication; this project would not have been possible without them. This research was supported by the following grants from National Institute on Drug Abuse: Johns Hopkins University (R01 DA015764) and the University of Vienna (R01DA018417).
Footnotes
Conflict of interest statement: None to declare.
Clinical trial registration details: International clinical trials registry platform, main ID: NCT 00271219
References
- 1.Goodrum L, Hankins G, Jermain D, Chanaud C. Conference Report: Complex Clincial, Legal, and Ethical Issues of Pregnant and Postpartum Women as Subjects in Clinical Trials. J Womens Health. 2003;12(9):835–837. doi: 10.1089/154099903770948087. [DOI] [PubMed] [Google Scholar]
- 2.Chervenak FA, McCullough LB. Ethics research and the pregnant patient. Curr Womens Health Rep. 2003;3(6):505–9. [PubMed] [Google Scholar]
- 3.Selby P, Kapur B, Hackman R, Koren G. “No one asked the baby”–An ethical issue in placebo-controlled trials in pregnant smokers. Can J Clin Pharmacol. 2005;12(2):e180–181. [PubMed] [Google Scholar]
- 4.McCullough LB, Coverdale JH, Chervenak FA. A comprehensive ethical framework for responsibly designing and conducting pharmacologic research that involves pregnant women. Am J Obstet Gynecol. 2005;193(3 Pt 2):901–7. doi: 10.1016/j.ajog.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 5.US Congress. National Institute of Health Revitalization Amendment. Public Law 103– 43. 6-10-1993
- 6.Winklbaur B, Kopf N, Ebner N, Jung E, Thau K, Fischer G. Treating pregnant women dependent on opioids is not the same as treating pregnancy and opioid dependence: a knowledge synthesis for better treatment for women and neonates. Addiction. 2008;103:1429–1440. doi: 10.1111/j.1360-0443.2008.02283.x. [DOI] [PubMed] [Google Scholar]
- 7.Jones HE, Johnson RE, Jasinski DR, O’Grady KE, Chisholm CA, Choo RE, et al. Buprenorphine versus methadone in the treatment of pregnant opioid-dependent patients: effects on the neonatal abstinence syndrome. Drug Alcohol Depend. 2005;79:1–10. doi: 10.1016/j.drugalcdep.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Fischer G, Ortner R, Rohrmeister K, Jagsch R, Baewert A, Langer M, et al. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101:275–81. doi: 10.1111/j.1360-0443.2006.01321.x. [DOI] [PubMed] [Google Scholar]
- 9.Fischer G, Johnson RE, Eder H, Jagsch R, Peternell A, Weninger M, et al. Treatment of opioid-dependent pregnant women with buprenorphine. Addiction. 2000;95:239–244. doi: 10.1046/j.1360-0443.2000.95223910.x. [DOI] [PubMed] [Google Scholar]
- 10.Johnson RE, Jones HE, Jasinski DR, Svikis DS, Haug NA, Jansson LM, et al. Buprenorphine treatment of opioid dependent women: maternal and neonatal outcomes. Drug Alcohol Depend. 2001;63:97–103. doi: 10.1016/s0376-8716(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 11.Choo R, Huestis M, Schroeder J, Shin A, Jones HE. Neonatal abstinence syndrome in methadone-exposed infants is altered by level of prenatal tobacco exposure. Drug Alcohol Depend. 2004;75:253–60. doi: 10.1016/j.drugalcdep.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Jones HE, Martin PR, Heil SH, Kaltenbach K, Selby P, Coyle MG, et al. Treatment of opioid-dependent pregnant women: clinical and research issues. J Subst Abuse Treat. 2008;35(3):245–259. doi: 10.1016/j.jsat.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unger A, Martin P, Kaltenbach K, Stine S, Heil S, Jones H, et al. Clinical Characteristics of Central European and North American Samples of Pregnant Women Screened for Opioid Agonist Treatment. Eur Addict Res. 2010;16:99–107. doi: 10.1159/000284683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stine SM, Heil SH, Kaltenbach K, Martin PR, Coyle MG, Fischer G, Aarria AM, Selby P, Jones HE. Characteristics of opioid-using pregnant women who accept or refuse participation in a clinical trial: screening results from the MOTHER study. Am J Drug Alcohol Abuse. 2009;35(6):429–33. doi: 10.3109/00952990903374080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones HE, Kaltenbach K, Heil S, Stine S, Coyle M, Arria A, O’Grady K, Selby P, Martin P, Fischer G. Neonatal Abstinence Syndrome Following Methadone or Buprenorphine Exposure. NEJM. 2010;363(24):2320–2341. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I disorders. New York: Biometrics Research, New York State Psychiatric Institute; 1996. [Google Scholar]
- 17.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 18.Jones HE, Harrow C, O’Grady KE, Crocetti M, Jansson LM, Kaltenbach K. Neonatal abstinence scores in opioid-exposed and nonexposed neonates: A blinded comparison. Journal of Opioid Management. doi: 10.5055/jom.2010.0038. (in press) [DOI] [PubMed] [Google Scholar]
- 19.Finnegan L, Kaltenbach K. Studies of prenatal drug exposure and environmental research issues: the benefits of integrating research within a treatment program. NIDA Res Monogr. 1992;117:259–70. [PubMed] [Google Scholar]
- 20.Jones HE, Haug N, Silverman K, Stitzer M, Svikis D. The effectiveness of incentives in enhancing treatment attendance and drug abstinence in methadone-maintained pregnant women. Drug Alcohol Depend. 2001;61:297–306. doi: 10.1016/s0376-8716(00)00152-6. [DOI] [PubMed] [Google Scholar]
- 21.Terplan M, Lui S. Psychosocial interventions for pregnant women in outpatient illicit drug treatment programs compared to other interventions. Cochrane Database of Systematic Reviews. 2007;4:CD006037. doi: 10.1002/14651858.CD006037.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher based re-enforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- 23.Winklbaur B, Baewert A, Jagsch R, Rohrmeister K, Metz V, Aeschbach Jachmann C, et al. Association between Prenatal Tobacco Exposure and Outcome of Neonates Born to Opioid-Maintained Mothers Implications for Treatment. Eur Addict Res. 2009;15:150–156. doi: 10.1159/000216466. [DOI] [PubMed] [Google Scholar]
- 24.Jones HE, Heil SH, O’Grady KE, Martin PR, Kaltenbach K, Coyle MG, Stine SM, Selby P, Aarria AM, Fischer G. Smoking in pregnant women screened for an opioid agonist medication study compared to related pregnant and non-pregnant samples. Am J Drug Alcohol Abuse. 2009;35(5):375–80. doi: 10.1080/00952990903125235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LeJeune C, Simmat-Durand L, Gourarier L, Aubisson S Groupe d’Etudes Grossesse et Addictions (GEGA) Prospective multicenter observational study of 260 infants born to 259 opiate-dependent mothers on methadone or high-dose buprenorphine substitution. Drug Alcohol Depend. 2006;82(39):250–7. doi: 10.1016/j.drugalcdep.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Ebner N, Rohrmeister K, Winklbaur B, Baewert A, Jagsch R, Peternell A, et al. Management of neonatal abstinence syndrome in neonates born to opioid maintained women. Drug Alcohol Depend. 2007;87(2–3):131–8. doi: 10.1016/j.drugalcdep.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 27.Kakko J, Heilig M, Sarman I. Buprenorphine and methadone treatment of opiate dependence during pregnancy: Comparison of fetal growth and neonatal outcomes in two consecutive case series. Drug Alcohol Depend. 2008;96:69–78. doi: 10.1016/j.drugalcdep.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 28.Bakstad B, Sarfi M, Welle-Strand GK, Ravndal E. Opioid maintenance treatment during pregnancy: occurrence and severity of neonatal abstinence syndrome. A national prospective study. Eur Addict Res. 2009;15(3):128–34. doi: 10.1159/000210042. [DOI] [PubMed] [Google Scholar]
- 29.Marquet P, Lavignasse P, Gautier PM. Case study of neonates born to mothers undergoing buprenorphine maintenance treatment. In: Kintz P, Marquet P, editors. Buprenorphine Therapy of Opiate Addiction. Totowa: Human Press; 2002. pp. 23–35. [Google Scholar]
- 30.Lim S, Prasad M, Samuels P, Gardner D, Cordero L. High-dose methadone in pregnant women and its effect on duration of neonatal abstinence syndrome. Am J Obstet Gyn. 2009;200(1):70.e1–70.e5. doi: 10.1016/j.ajog.2008.08.041. [DOI] [PubMed] [Google Scholar]
- 31.Kahila H, Saisto T, Kivitie-Kallio S, Haukkamaa M, Halmesmaki E. A prospective study on buprenorphine use during pregnancy: effects on maternal and neonatal outcome. Acta Obstet Gyn Scan. 2007;86:185–190. doi: 10.1080/00016340601110770. [DOI] [PubMed] [Google Scholar]
- 32.Schindler S, Eder H, Ortner R, Rohrmeister K, Langer M, Fischer G. Neonatal outcome following buprenorphine maintenance during conception and throughout pregnancy. Addiction. 2003;98:103–110. doi: 10.1046/j.1360-0443.2003.00245.x. [DOI] [PubMed] [Google Scholar]
- 33.Cleary B, Donnelly J, Strawbridge J, Gallagher PJ, Fahey T, Clarke M, Murphy DJ. Methadone dose and neonatal abstinence syndrome - systematic review and meta-analysis. Addiction. 2010 doi: 10.1111/j.1360-0443.2010.03120.x. [DOI] [PubMed] [Google Scholar]
- 34.Fischer G. Treatment of opioid dependence in pregnant women. Addiction. 2000;95(8):1141–1144. doi: 10.1046/j.1360-0443.2000.95811411.x. [DOI] [PubMed] [Google Scholar]
- 35.Fischer G. Our ethical responsibility. Addiction. 2000;95(12):1769–70. [PubMed] [Google Scholar]


