Table 1.
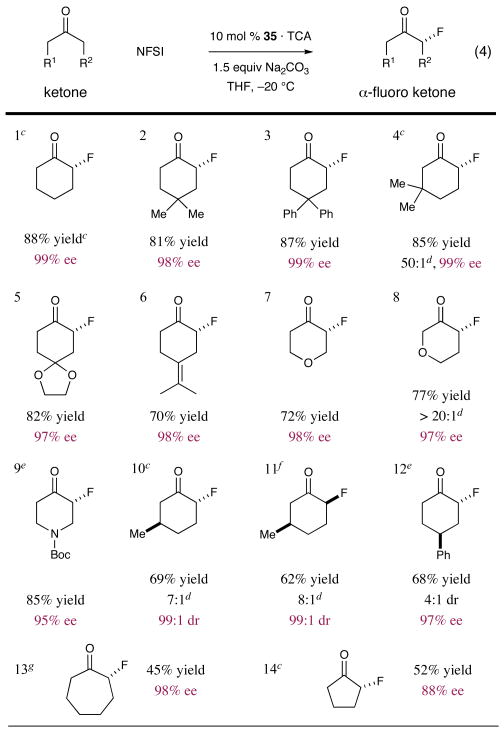 |
Isolated yield.
Determined by chiral GC-FID, absolute configuration determined by chemical correlation, X-ray analysis or by analogy.
GC yield.
Regioselectivity.
Using 20 mol % 35·TCA.
Using 10 mol % epi-34·TCA.
The difluorination product was obtained in 34% yield.
