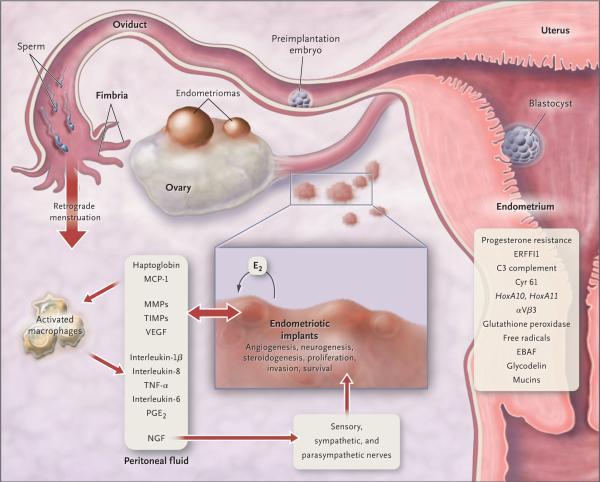
Figure 2. Pathophysiology of Pain and Infertility Associated with Endometriosis.
Retrograde transplanted endometrial tissue and cells attach to peritoneal surfaces, establish a blood supply, and invade nearby structures. They are infiltrated by sensory, sympathetic, and parasympathetic nerves and elicit an inflammatory response. Endometriotic implants secrete estradiol (E2) as well as prostaglandin E2 (PGE2), agents that attract macrophages (monocyte chemotactic protein 1 [MCP-1]), neurotrophic peptides (nerve growth factor [NGF]), enzymes for tissue remodeling (matrix metalloproteinases [MMPs]) and tissue inhibitors of MMPs (TIMPs), and proangiogenic substances such as vascular endothelial growth factor (VEGF) and interleukin-8. Lesions secrete haptoglobin, which decreases macrophage adhesion and phagocytic function. Lesions and activated macrophages, which are abundant in the peritoneal fluid in women with endometriosis, also secrete proinflammatory cytokines (interleukin-1β, interleukin-8, interleukin-6, and tumor necrosis factor α [TNF-α]). Local (and systemic) estradiol can stimulate lesion production of PGE2, which can activate pain fibers, enhance neuronal invasion of lesions by stimulating production of NGF and other neurotrophins, and promote sprouting of nociceptors that contribute to persistent inflammatory pain and inhibit neuronal apoptosis. Endometrial bleeding factor (EBAF) is misexpressed and may contribute to uterine bleeding. Infertility results from the toxic effects of the inflammatory process on gametes and embryos, compromised fimbrial function, and eutopic endometrium that is resistant to the action of progesterone and is inhospitable to embryonic implantation. HoxA10 and HoxA11 genes and αVβ3 integrin are not up-regulated by progesterone, and thus the endometrium is inhospitable to an implanting embryo. Endocrine-disrupting chemicals can contribute to progesterone resistance and perhaps immune dysfunction.1,4 ERFFI1 (ErbB receptor feedback inhibitor 1) is constitutively expressed and there is excess mitogenic signaling.