Figure 6.
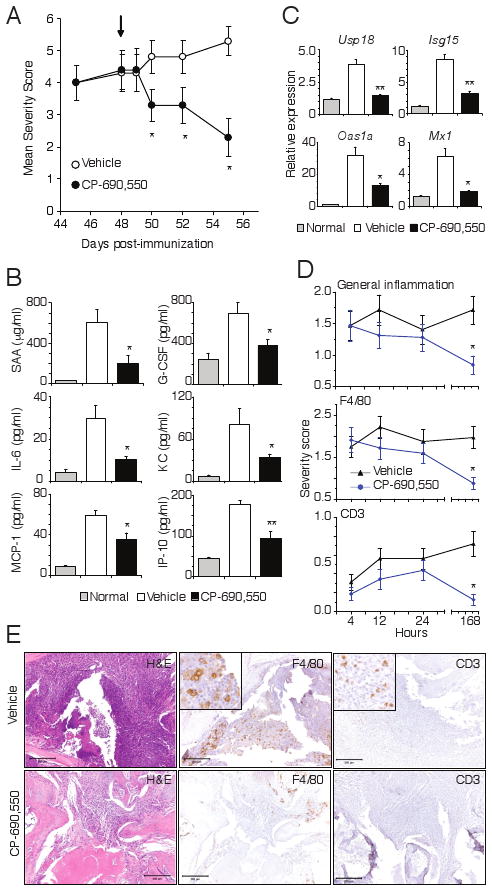
Rapid amelioration of established arthritis and inflammation by CP-690,550. Mice with fully developed CIA were orally administrated vehicle of CP-690,550 at 50 mg/kg b.i.d. beginning on day 48 post immunization (arrow). Data represent the mean ± SEM severity score from 8 mice in each group (A). Plasma concentrations of SAA, G-CSF, IL-6, CXCL1 (KC), CCL2 (MCP-1) and CXCL10 (IP-10) were measured 4 h after dosing on day 48 (B). Relative expression of STAT1-induced genes in paw tissue 4 h after dosing on day 48 (C). Usp18, ubiquitin specific peptidase 18; Isg15, interferon-stimulated gene 15; Oas1a, 2′-5′ oligoadenylate synthase 1A; Mx1, myxovirus resistance protein 1. Quantitative RT-PCR results were normalized to cyclophilin and represent mean ± SEM relative expression from non-diseased animals (normal), as well as vehicle and CP-690,550 treated mice with CIA (C). Cellular infiltration of inflamed paw tissue in mice treated as in A was assessed by histology and IHC at the indicated time points (D). Data represents the mean ± SEM score for all 4 paws from 8 mice per treatment group. For all panels, *P<0.01 and **P<0.001. Representative histology (H&E) and F4/80 or CD3 IHC of paw tissue sections collected 7 days after onset of treatment with vehicle or CP-690,550 (E).
