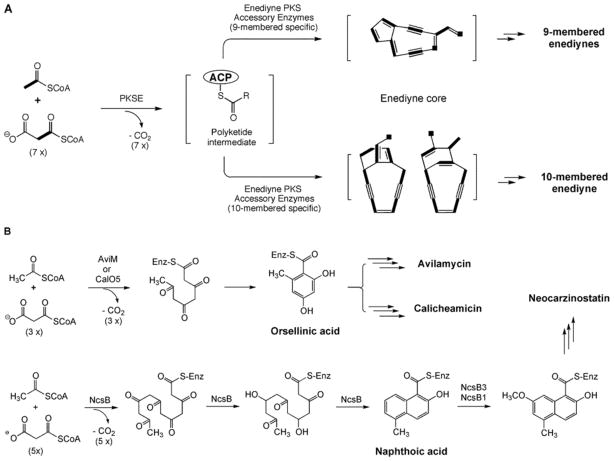
Fig. 5.
(A) Proposed pathway for biosynthesis of a polyunsaturated intermediate (structure unknown) from acyl CoA by PKSE and the subsequent transformation by enediyne PKS associated enzymes into putative nine- or ten-membered enediyne cores that are finally tailored to individual enediyne natural product. Atoms that were incorporated intact from acyl CoA precursors to the enediyne cores are shown in bold. (B) Proposed pathway for the biosynthesis of aromatic polyketides by iterative type I PKS as exemplified by AviM or CalO5 for orsellinic acid and by NcsB for a naphthoic acid.