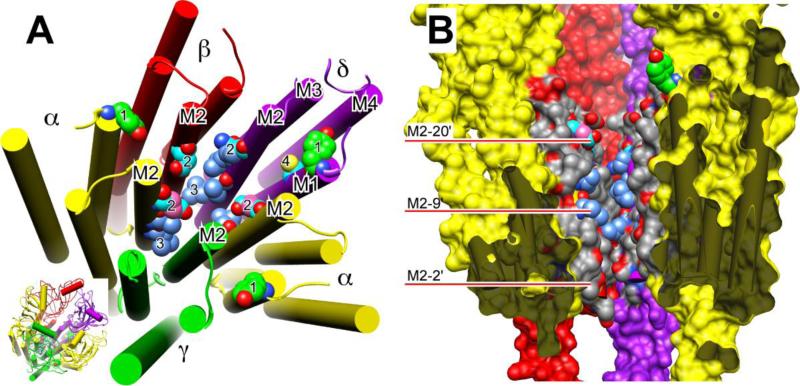
Figure 4.
Residues photolabeled on nicotinic receptors by three classes of general anesthetics. Panel A shows a slightly tilted top view of just the transmembrane domain of the nAChR of the nicotinic acetylcholine receptor (nAChR) from Torpedo using the cryoelectron microscopy structure (20). The agonist-binding domain has been omitted for clarity, but is shown in the inset at bottom right, where the orientation is the same as in the larger diagram. The subunits are color-coded and labeled as in Fig. 2. The helices are shown as rods and the atoms of photolabeled residues, including the backbone atoms, are shown in space-filled mode. All other residues are omitted for clarity. Oxygen (red) and nitrogen (blue) atoms are colored conventionally. The carbon atoms are color coded to denote which anesthetics photolabeled the residue: green, halothane; cyan, azietomidate; cornflower blue, TDBzl-etomidate; salmon, azioctanol. In some cases, more than one agent photolabels the same residue and individual carbons are given different colors accordingly. Number code: 1. αY213 & δY228 photolabeled on M1 by halothane; 2. αE262, βD268 & δQ276 (the M2-20' residues) photolabeled by azietomidate and azioctanol; 3. αL251, δL265 (the M2-9' residues) photolabeled by TDBzl-etomidate; 4. δC236 on M1 Photolabeled by azietomidate. Panel B shows a cross-section through the transmembrane domain of the same nAChR structure. The γ-subunit has been removed to facilitate a view of the ion pore. The viewer is situated at the γ-subunit and the two α-subunits are closest to the viewer. The subunits have the same color code as before but are presented surfaced. The dark grey regions denote where the surface of the α-subunits have been cut through and serve to emphasize the free space that exists within the 4-helix bundle of subunits. The central ion channel is open to view. The M2 helices are colored conventionally with grey for carbon, red for oxygen and blue for nitrogen, except that in photolabeled residues some of the carbons have the same color code as in panel A. The red bars point to residues on M2 using the prime numbering system, where 1' is the residue following the last charged residue before the M2 helix and M2-9' is always the conserved leucine.