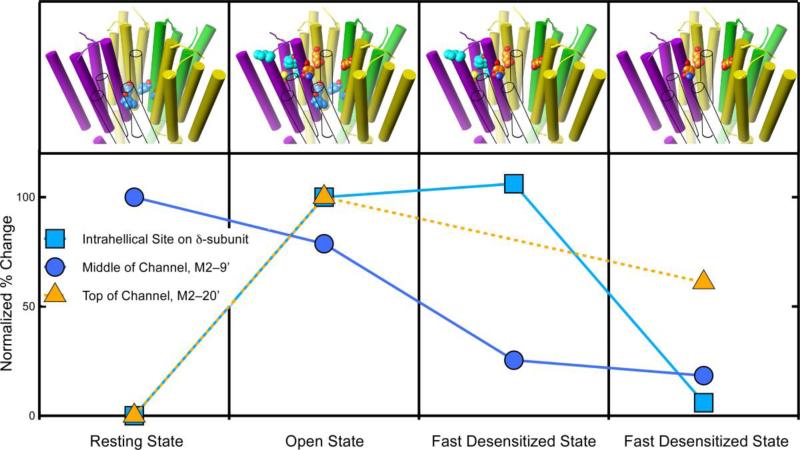
Figure 6.
The degree of photolabeling of three sites on the nAChR varies with the receptor's conformation, supporting allosteric action. The graph shows the relative level of photoincorporation for three different sites on the nicotinic acetylcholine receptor. The photoincorporation level is normalized to that first observed (either the resting or the open state). The upper panel depicts the transmembrane domain of the nAChR and the photolabeled residues in each state. The subunit colors are yellow for α, green for γ, and purple for δ. The β-subunit is shown in outline only to allow the channel residues to be seen. The carbon atoms are colored with the same color as the symbols on the graph. At two sites photolabeling is negligible in the resting state but increases dramatically when the channel opens. One of these sites (orange triangles) is in the upper part of the channel (M2–20') and the other (cyan squares) is in the intrahelical bundle of the β-subunit. The behavior of these sites diverges during desensitization. The channel site changes modestly, whereas the intrahelical site remains unchanged during fast desensitization and then decreases dramatically upon slow desensitization. The third site (blue circles) is also in the channel but at the conserved M2–9' leucines. It behaves differently from the site in the upper part of the channel. It is photolabeled in the resting state and in the open state, but photolabeling decreases dramatically during fast desensitization and remains unchanged during slow desensitization. Data for azietomidate (triangles) is from (40) and for TID (circles and squares) is from (52, 53).