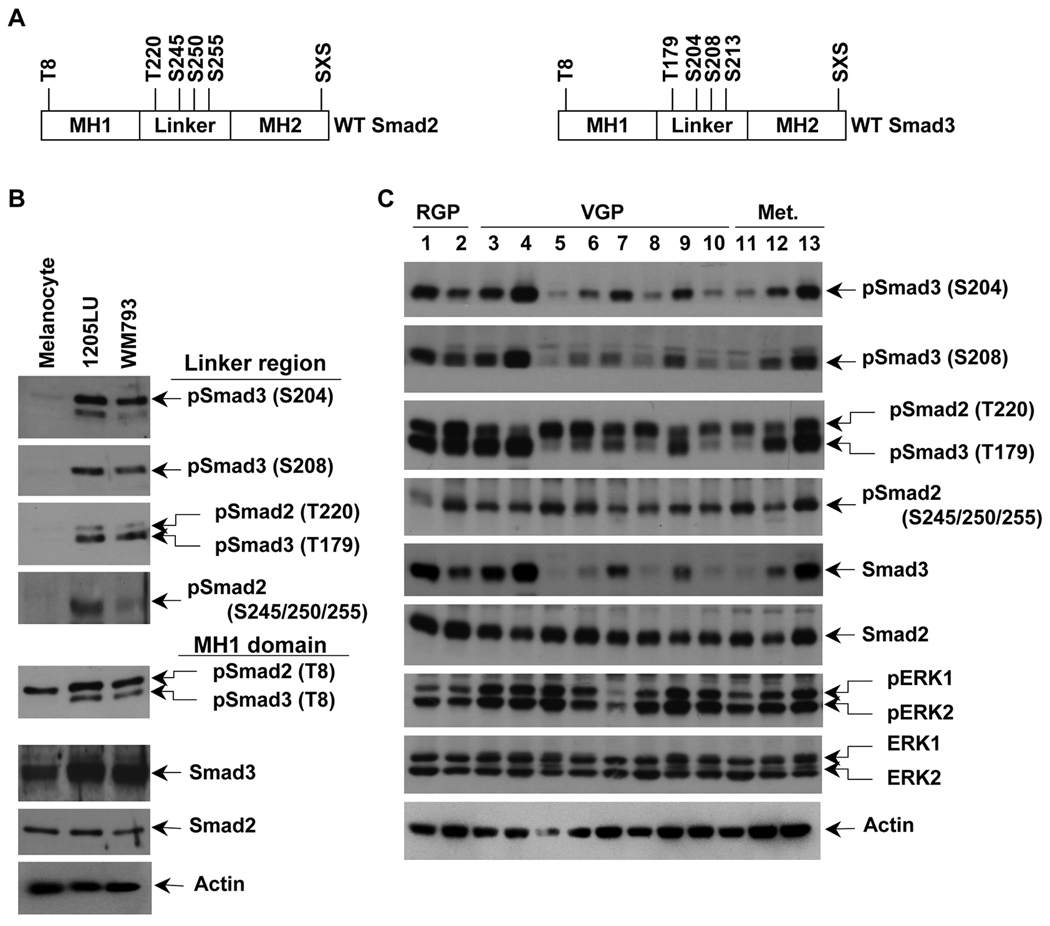
Figure 2. Constitutive linker phosphorylation of Smad2 and Smad3 in human melanoma lines.
A. Schematic representation of wild type Smad2 and Smad3. Smad2 has one phosphorylation site, threonine 8, in the MH1 domain (N-terminal domain), four phosphorylation sites in the linker region, threonine 220, serines 245, 250 and 255, and the SXS motif in the MH2 (C-terminal) domain. Smad3 has one phosphorylation site, threonine 8, in the MH1 domain (N-terminal domain), four phosphorylation sites in the linker region, threonine 179, serines 204, 208 and 213, and the SXS motif in the MH2 domain. B. High constitutive phosphorylation of Smad2 and Smad3 at the linker region in WM793 and 1205LU melanoma lines and not in melanocytes. The two melanoma lines, 1205LU and WM793, and the melanocytes were serum-starved overnight, before protein extraction. Immunoblot experiments using polyclonal antibodies against: Both phosphoSmad3 (Thr179) and phosphoSmad2 (Thr220); phosphoSmad2 (Ser245/250/255); phosphoSmad3 (Ser204); phosphoSmad3 (Ser208); both phosphoSmad3 (Thr8) and phosphoSmad2 (Thr8); total Smad3; total Smad2. C. Constitutive linker phosphorylation of Smad2 and Smad3 in a panel of human melanoma cell lines from different melanoma stages. The human melanoma lines were derived from Radial Growth Phase (RGP): 1: WM35; 2: 1552c; Vertical Growth Phase (VGP): 3: WM793; 4: WM278; 5: A2058; 6: SKMEL2; 7: SKMEL5; 8: SKMEL28; 9: SKMEL31; 10: HT144; primary melanomas and metastatic (Met.) melanomas: 11: WM1617; 12: C8161; 13: 1205LU. Cells were serum-starved overnight, before protein extraction as in B. p: phospho. S: Ser; T: Thr. Actin expression was used as a control.