Abstract
Upon DNA double-strand break (DSB) formation, hundreds of H2AX molecules in the chromatin flanking the break site are phosphorylated on serine residue 139, termed gamma-H2AX, so that virtually every DSB site in a nucleus can be visualised within 10 min of its formation using an antibody to gamma-H2AX. One application of this sensitive assay is to examine the induction of DNA double-strand damage in subtle non-targeted cellular effects such as the bystander effect. Here whether microRNA (miRNA) serve as a primary signalling mechanism for bystander effect propagation by comparing matched human colon carcinoma cell lines with wild-type or depleted levels of mature miRNAs was investigated. No major differences were found in the levels of induced gamma-H2AX foci in the tested cell lines, indicating that though miRNAs play a role in bystander effect manifestation, they appear not to be the primary bystander signalling molecules in the formation of bystander effect-induced DSBs.
INTRODUCTION
The radiation-induced bystander effect (RIBE) constitutes a phenomenon whereby unexposed naïve cells that were either in direct contact with irradiated cells (IR) or received media-borne signals from IR also exhibit cellular damage(1–5). The RIBE induces a wide array of genetic alterations including gross genome rearrangements, chromosome aberrations, sister chromatid exchanges, deletions, duplications and gene mutations, which may result in altered gene expression, changes in cellular proliferation, senescence and cell death(6–15). Thus, though the RIBE is a well-accepted outcome of radiation exposure, the nature of bystander signalling remains unclear.
However, one early response to bystander signalling is the formation of DNA double-strand breaks (DSBs), the level of which can be assayed by the formation of gamma-H2AX foci, resulting from the phosphorylation of hundreds of H2AX molecules in the chromatin flanking the break sites(16, 17). Because of this amplified response, virtually every DSB site in a nucleus can be visualised within 10 min of its formation using an antibody to phosphorylated H2AX (gamma-H2AX)(17). This study utilises this sensitivity to ask whether small regulatory RNAs [specifically microRNAs (miRNAs)] may be involved in bystander signalling.
We hypothesised that bystander effects might be manifested through miRNA involvement. MiRNAs are small and relatively stable molecules, characteristics consistent with their being plausible candidates for primary bystander signals. In addition, profound deregulation of the microRNAome occurs in bystander cells (BS) with a significant correlation between miRNA expression and levels of their target proteins(18–20).
To examine this hypothesis, Dicer knockdown cell lines were utilised to determine if bystander effects were affected by decreased miRNA signalling. Dicer cleaves pre-miRNAs as they are exported from the nucleus to produce mature miRNAs(21, 22). In the absence of Dicer, functional miRNAs cannot be manufactured. DNA DSB formation, an early and critical aspect of RIBE signalling, to monitor bystander responses was measured(14, 15, 23). Results obtained from counting gamma-H2AX foci by eye were compared with those obtained from a commercial software program (see Experimental Methods section).
Bystander effect signalling was monitored in two distinct systems. One protocol utilised the transfer of conditioned media from cultures exposed to ionising radiation to other unexposed, bystander cultures(23). The second protocol compared shielded (bystander) and unshielded (exposed) portions of sensitised cultures after exposure to UVA light(24). These two very different techniques have both been shown to induce bystander DNA DSBs in unirradiated cell culture samples(24, 25), indicating that RIBE and other bystander effects may all be specific instances of a general cellular stress response that is propagated through similar signalling pathways.
EXPERIMENTAL METHODS
Matched human colon carcinoma cell lines with wild-type or depleted levels of Dicer were the kind gift of Bert Vogelstein, Johns Hopkins University School of Medicine, and have been described previously(26). Cells were seeded in two-well Lab-Tek chamber slides (Nunc, Naperville, IL, USA) 2 days before the experiment and grown to 75–80 % confluence. After being subjected to either of two bystander protocols (described below), the cells were fixed in 2 % paraformaldehyde in phosphate buffered saline (PBS), washed and permeabilised in ethanol. Preparations were blocked in bovine serum albumin and incubated with the anti-gamma-H2AX rabbit antibody (custom-made) and the anti-rabbit Alexa-555-labelled antibody (Molecular Probes, Eugene, OR, USA). The slides were then washed, mounted with 4′6-diaidino-2-phenylindole (DAPI) and viewed as previously described(15).
Foci were counted by eye and by focal identification software. Manual counting of foci by eye is relatively straightforward, but can become burdensome and may introduce a risk of bias. Therefore, in this study, we compared the results of counting by eye with counting by a BD Pathway Bioimager coupled to the IP Labs software package to automatically determine gamma-H2AX focal number, size and intensity in all samples. The results of this comparison indicate that software analysis may automate many of the steps involved in gamma-H2AX detection, and in addition, minimise possible observer bias in determining DSB numbers. This ability is particularly useful in studies of bystander effects because they are often small.
Thus, gamma-H2AX intensity and focal numbers were quantified using maximal projections of z-stack images generated by a spinning-disc confocal BD Pathway Bioimager (BD Biosciences, San Diego, CA, USA). The IP Labs software (BD Biosciences) was then used to automatically determine gamma-H2AX foci number, size and intensity in at least three separate fields of ∼200 cells. In order to determine the significance of the difference between means, a Student's t-test was used. Analysis was performed using the JMP 5.0 and Excel XP software (Microsoft Corp., Redmond, WA, USA).
Bystander protocol: media transfer
Each cell line was split into three multi-well slides and grown overnight. Two slides were exposed to 2.5 Gy of X rays and incubated for various lengths of time. The media from unirradiated slides were discarded and replaced with media from one of the irradiated slides. After incubation for various times, the slides were processed for gamma-H2AX focal analysis.
Bystander protocol: co-culture
The bystander effect was initiated by DSB induction in targeted cells using the photolysis methodology described by Limoli and Ward(27). Characterisation of bystander effects induced through this method was described previously(24). Briefly, bromodeoxyuridine (BrdU Sigma, St. Louis, MO, USA) was added to the cell medium at a final concentration of 10 μM and allowed to incorporate into cells overnight. Hoechst dye 33342 (10 μg, Sigma) was added to the cell medium 20 min prior to the experiment. Following Hoechst dye incorporation, the medium was aspirated, the cells were washed with PBS and 200 μl of cold medium was added to the surface of the cells. The cells were placed on ice, and aluminium foil was used to shield half of each culture. The cells were exposed to a final dose of 0.04 J cm−2 for a total of 1 min using a Blak-Ray Longwave Ultraviolet Lamp (UVP, San Gabriel, CA, USA). Fresh media was added to the cells, and they were allowed to incubate from 30 min to overnight before processing for gamma-H2AX focal analysis.
RESULTS AND DISCUSSION
Matched human colon carcinoma cell lines with wild-type or depleted levels of Dicer were used in this study. These cells were previously reported to have largely reduced amounts of mature miRNAs(26) and are used in lieu of knockout cell lines that are non-viable in vertebrate cells(26). These knockdown lines were derived from three colorectal cell lines (HCT-116, DLD-1 and RKO), displaying a hypomorphic phenotype of Dicer that stemmed from a disruption of exon 5. Importantly, all three Dicer exon 5-disrupted lines have reduced amounts of mature miRNAs and increased miRNA precursors when compared with their corresponding parental lines.
Dicer knockdown cell lines exhibit bystander effects
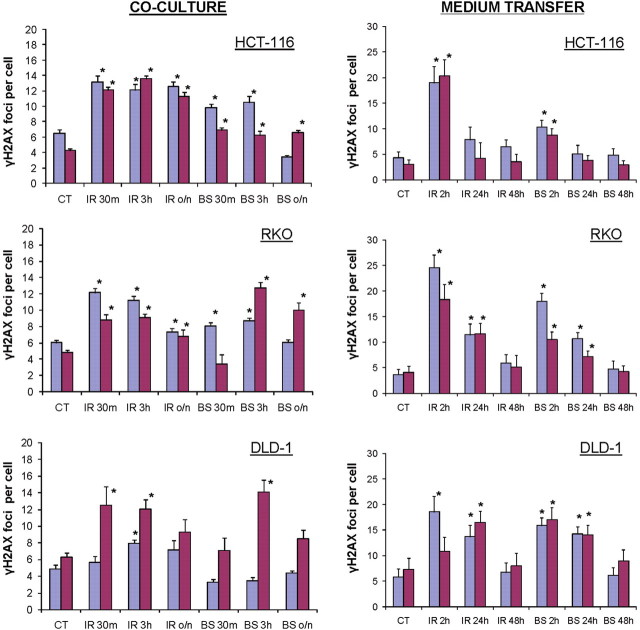
Two different protocols were used for the induction of RIBE; medium transfer and co-culture(23) and examined a well-characterised RIBE endpoint; the induction of gamma-H2AX foci which mark DNA DSBs(28). The results show that depletion of miRNA levels in the HCT-116, RKO and DLD-1 carcinoma cell lines did not abrogate their capabilities to exhibit bystander effects (Figure 1). Elevated levels of gamma-H2AX foci were apparent at 2 h in the medium transfer experiments (left panels) and 30 min in the co-culture experiments (right panels) in the BS as well as the IR.
Figure 1.
Levels of gamma-H2AX foci in irradiated and bystander wild-type and Dicer knockdown cell lines. Bystander effect was induced by co-culture or medium transfer techniques. Blue bars, wild-type cells; Purple bars, Dicer knockdown cells. Co-culture: CT, control; IR, irradiated; 30m, 30 minutes; 3h, 3 hours; BS, bystander cells; o/n, overnight incubation. Medium transfer: CT, control; IR, irradiated; 2h, 2 hours; 24h, 24 hours; 48h, 48 hours; BS, bystander cells. *, significantly different from control, p < 0.05, t-test.
While differences in the responses of the three different matched pairs of cell lines to the media transfer protocol were observed, the results show that the cell lines with depleted miRNA levels exhibited bystander responses very similar to their wild-type counterparts. The results were more variable in the co-culture protocol. In two of the matched pairs of cell lines, RKO and DLD-1, the miRNA-depleted cells appeared to exhibit somewhat greater bystander responses than their wild-type partners. However, these results may be due to the greater complexity of the co-culture protocol, which depends on BrdU being incorporated into the cellular DNA. Thus different responses of the matched pairs of cell lines may be due in part to more variable extents of BrdU uptake. However, the results do show that in each of these cell line pairs, the lines with depleted miRNA levels exhibits as strong a co-culture bystander effect as does their wild-type partner.
Computer aided computation of gamma-H2AX focal numbers
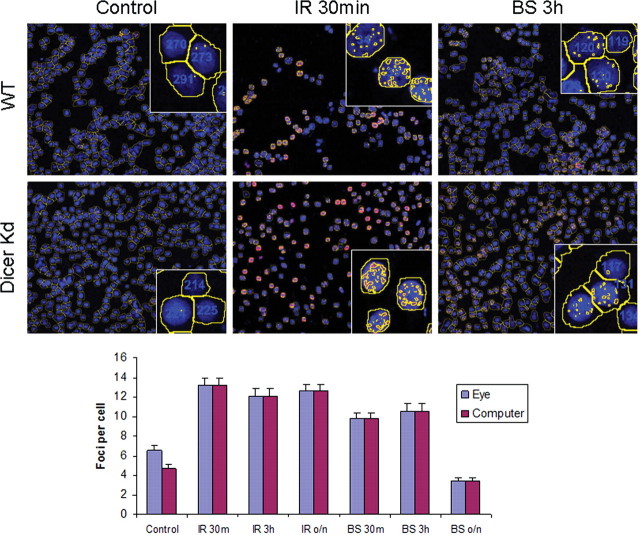
In these experiments, gamma-H2AX foci were counted by eye on maximum projections of z-stacked images captured by spinning-disc confocal microscopy. While these counts are reliable if performed in a blinded fashion by two independent observers, an instrument-based approach has several advantages, including more rapid processing and less chance for unconscious observer bias. However, software programs also have their weaknesses, often in the initial recognition of gamma-H2AX foci against various backgrounds. The capabilities of IP Labs automatic counting software on the same slides and same microscopic fields that had been analysed visually were compared. The top panel of Figure 2 shows representative images of HCT-116 WT and Dicer knockdown cells. Control cells as well as directly irradiated samples after 30 min and BS after 3 h are shown. The yellow lines around the DAPI stained nuclei indicate how the computer program was able to differentiate between cell, and foci within the nuclei were highlighted with yellow and counted. Inset images show three individual cells within the field and how they were counted. In all cases, the data obtained from computer-aided analysis was nearly identical to that obtained through manual counting (Figure 2, bottom panel). Additionally, as seen from these images there was very little difference in the DNA DSB response to bystander signalling in these cell populations. These results indicate that future studies could be performed using this automated counting programme, allowing results to be obtained more quickly and without any pre-conditioned bias.
Figure 2.
Automated foci counting performed by the IP Labs software. Top: HCT-116 cells from the co-culture experiment are shown. WT, top row, wild-type cells; Dicer Kd, bottom row, Dicer knockdown cells. Blue DAPI stained nuclei are shown, gamma-H2AX foci are stained in red but yellow spots indicate that the foci were seen and counted by the IP Labs software. Likewise, yellow outlines were generated by the software to distinguish individual cell nuclei. Bottom: The gamma-H2AX foci per cell were counted in wild-type HCT-116 cells either manually by eye (blue bars) or using the IP Labs computer software (red bars). The error bars represent the SEM for ∼200 cells in three separate microscopic fields.
CONCLUSIONS
Overall, the magnitude of the bystander gamma-H2AX response was similar in wild-type and Dicer knockdown paired carcinoma cell lines. These results show that partial miRNA depletion does not abrogate the generation or maintenance of bystander responses, and indicate that it is unlikely that miRNAs play primary roles in mediating bystander effects. However, since mammalian cell lines null for Dicer are not viable, and since small amounts of miRNAs is still present in Dicer knockdown cells, future studies on the roles of miRNAs in bystander signalling are required.
FUNDING
Funding for this research was provided by the intramural research program of the National Cancer Institute (J.S.D., O.A.S., W.M.B.), and the Alberta Cancer board operating grant (F.J.Z., A.A., O.K.).
REFERENCES
- 1.Morgan W. F. Is there a common mechanism underlying genomic instability, bystander effects and other nontargeted effects of exposure to ionizing radiation? Oncogene. 2003;22:7094–7099. doi: 10.1038/sj.onc.1206992. doi:10.1038/sj.onc.1206992. [DOI] [PubMed] [Google Scholar]
- 2.Morgan W. F. Non-targeted and delayed effects of exposure to ionizing radiation: II. Radiation-induced genomic instability and bystander effects in vivo, clastogenic factors and transgenerational effects. Radiat. Res. 2003;159:581–596. doi: 10.1667/0033-7587(2003)159[0581:nadeoe]2.0.co;2. doi:10.1667/0033-7587(2003)159[0581:NADEOE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Morgan W. F. Non-targeted and delayed effects of exposure to ionizing radiation: I. Radiation-induced genomic instability and bystander effects in vitro. Radiat. Res. 2003;159:567–580. doi: 10.1667/0033-7587(2003)159[0567:nadeoe]2.0.co;2. doi:10.1667/0033-7587(2003)159[0567:NADEOE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 4.Mothersill C., Seymour C. Radiation-induced bystander and other non-targeted effects: novel intervention points in cancer therapy? Curr. Cancer Drug. Targets. 2006;6:447–454. doi: 10.2174/156800906777723976. doi:10.2174/156800906777723976. [DOI] [PubMed] [Google Scholar]
- 5.Mothersill C., Seymour C. B. Radiation-induced bystander effects–implications for cancer. Nat. Rev. Cancer. 2004;4:158–164. doi: 10.1038/nrc1277. [DOI] [PubMed] [Google Scholar]
- 6.Zhou H., Randers-Pehrson G., Hall E. J., Brenner D. J., Geard C., Hei T. K. Interaction between radiation-induced adaptive response and bystander mutagenesis in mammalian cells. Radiat. Res. 2003;161:512–516. doi: 10.1667/rr3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou H., Randers-Pehrson G., Suzuki M., Waldren C. A., Hei T. K. Genotoxic damage in non-irradiated cells: contribution from the bystander effect. Radiat. Prot. Dosimetry. 2002;99:227–232. doi: 10.1093/oxfordjournals.rpd.a006769. [DOI] [PubMed] [Google Scholar]
- 8.Zhou H., Randers-Pehrson G., Waldren C. A., Vannais D., Hall E. J., Hei T. K. Induction of a bystander mutagenic effect of alpha particles in mammalian cells. Proc. Natl. Acad. Sci. USA. 2000;97:2099–2104. doi: 10.1073/pnas.030420797. doi:10.1073/pnas.030420797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klokov D., Criswell T., Leskov K. S., Araki S., Mayo L., Boothman D. A. IR-inducible clusterin gene expression: a protein with potential roles in ionizing radiation-induced adaptive responses, genomic instability, and bystander effects. Mutat. Res. 2004;568:97–110. doi: 10.1016/j.mrfmmm.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 10.Smilenov L. B., Hall E. J., Bonner W. M., Sedelnikova O. A. A microbeam study of DNA double-strand breaks in bystander primary human fibroblasts. Radiat. Prot. Dosimetry. 2006;122:256–259. doi: 10.1093/rpd/ncl461. doi:10.1093/rpd/ncl461. [DOI] [PubMed] [Google Scholar]
- 11.Lorimore S. A., Coates P. J., Scobie G. E., Milne G., Wright E. G. Inflammatory-type responses after exposure to ionizing radiation in vivo: a mechanism for radiation-induced bystander effects? Oncogene. 2001;20:7085–7095. doi: 10.1038/sj.onc.1204903. doi:10.1038/sj.onc.1204903. [DOI] [PubMed] [Google Scholar]
- 12.Mothersill C., Rea D., Wright E. G., Lorimore S. A., Murphy D., Seymour C. B., O'Malley K. Individual variation in the production of a ‘bystander signal’ following irradiation of primary cultures of normal human urothelium. Carcinogenesis. 2001;22:1465–1471. doi: 10.1093/carcin/22.9.1465. doi:10.1093/carcin/22.9.1465. [DOI] [PubMed] [Google Scholar]
- 13.Lyng F. M., Maguire P., McClean B., Seymour C., Mothersill C. The involvement of calcium and MAP kinase signaling pathways in the production of radiation-induced bystander effects. Radiat. Res. 2006;165:400–409. doi: 10.1667/rr3527.1. doi:10.1667/RR3527.1. [DOI] [PubMed] [Google Scholar]
- 14.Sokolov M. V., Dickey J. S., Bonner W. M., Sedelnikova O. A. gamma-H2AX in bystander cells: not just a radiation-triggered event, a cellular response to stress mediated by intercellular communication. Cell Cycle. 2007;6:2210–2212. doi: 10.4161/cc.6.18.4682. doi:10.4161/cc.6.18.4682. [DOI] [PubMed] [Google Scholar]
- 15.Sedelnikova O. A., Nakamura A., Kovalchuk O., Koturbash I., Mitchell S. A., Marino S. A., Brenner D. J., Bonner W. M. DNA double-strand breaks form in bystander cells after microbeam irradiation of three-dimensional human tissue models. Cancer Res. 2007;67:4295–4302. doi: 10.1158/0008-5472.CAN-06-4442. doi:10.1158/0008-5472.CAN-06-4442. [DOI] [PubMed] [Google Scholar]
- 16.Rogakou E. P., Pilch D. R., Orr A. H., Ivanova V. S., Bonner W. M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. doi:10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 17.Rogakou E. P., Boon C., Redon C., Bonner W. M. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell Biol. 1999;146:905–916. doi: 10.1083/jcb.146.5.905. doi:10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ilnytskyy Y., Koturbash I., Kovalchuk O. Radiation-induced bystander effects in vivo are epigenetically regulated in a tissue-specific manner. Environ. Mol. Mutagen. 2009;50:105–113. doi: 10.1002/em.20440. doi:10.1002/em.20440. [DOI] [PubMed] [Google Scholar]
- 19.Koturbash I., Zemp F. J., Kutanzi K., Luzhna L., Loree J., Kolb B., Kovalchuk O. Sex-specific microRNAome deregulation in the shielded bystander spleen of cranially exposed mice. Cell Cycle. 2008;7:1658–1667. doi: 10.4161/cc.7.11.5981. [DOI] [PubMed] [Google Scholar]
- 20.Koturbash I., Boyko A., Rodriguez-Juarez R., McDonald R. J., Tryndyak V. P., Kovalchuk I., Pogribny I. P., Kovalchuk O. Role of epigenetic effectors in maintenance of the long-term persistent bystander effect in spleen in vivo. Carcinogenesis. 2007;28:1831–1838. doi: 10.1093/carcin/bgm053. doi:10.1093/carcin/bgm053. [DOI] [PubMed] [Google Scholar]
- 21.Bassing C. H., Suh H., Ferguson D. O., Chua K. F., Manis J., Eckersdorff M., Gleason M., Bronson R., Lee C., Alt F. W. Histone H2AX: a dosage-dependent suppressor of oncogenic translocations and tumors. Cell. 2003;114:359–370. doi: 10.1016/s0092-8674(03)00566-x. doi:10.1016/S0092-8674(03)00566-X. [DOI] [PubMed] [Google Scholar]
- 22.Mack G. S. MicroRNA gets down to business. Nat. Biotechnol. 2007;25:631–638. doi: 10.1038/nbt0607-631. doi:10.1038/nbt0607-631. [DOI] [PubMed] [Google Scholar]
- 23.Sokolov M. V., Smilenov L. B., Hall E. J., Panyutin I. G., Bonner W. M., Sedelnikova O. A. Ionizing radiation induces DNA double-strand breaks in bystander primary human fibroblasts. Oncogene. 2005;24:7257–7265. doi: 10.1038/sj.onc.1208886. doi:10.1038/sj.onc.1208886. [DOI] [PubMed] [Google Scholar]
- 24.Dickey J. S., Baird B. J., Redon C. E., Sokolov M. V., Sedelnikova O. A., Bonner W. M. Intercellular communication of cellular stress monitored by gamma-H2AX induction. Carcinogenesis. 2009;30:1686–1695. doi: 10.1093/carcin/bgp192. doi:10.1093/carcin/bgp192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koturbash I., Rugo R. E., Hendricks C. A., Loree J., Thibault B., Kutanzi K., Pogribny I., Yanch J. C., Engelward B. P., Kovalchuk O. Irradiation induces DNA damage and modulates epigenetic effectors in distant bystander tissue in vivo. Oncogene. 2006;25:4267–4275. doi: 10.1038/sj.onc.1209467. doi:10.1038/sj.onc.1209467. [DOI] [PubMed] [Google Scholar]
- 26.Cummins J. M., et al. The colorectal microRNAome. Proc. Natl. Acad. Sci. USA. 2006;103:3687–3692. doi: 10.1073/pnas.0511155103. doi:10.1073/pnas.0511155103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Limoli C. L., Ward J. F. A new method for introducing double-strand breaks into cellular DNA. Radiat. Res. 1993;134:160–169. doi:10.2307/3578455. [PubMed] [Google Scholar]
- 28.Bonner W. M., Redon C. E., Dickey J. S., Nakamura A. J., Sedelnikova O. A., Solier S., Pommier Y. gammaH2AX and cancer. Nat. Rev. Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. doi:10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]