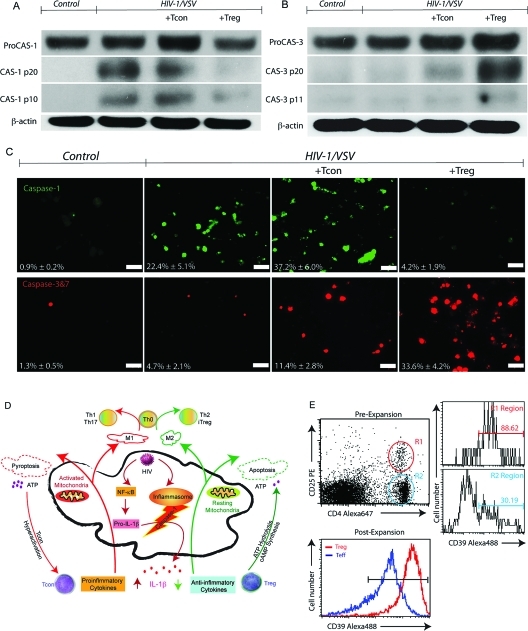
Figure 3.
Treg and Tcon Induced Virus-Infected BMM Apoptosis and Pyroptosis. (A) Western blotting showed HIV-1/VSV infection triggers caspase-1 activation in BMM, which was enhanced by Tcon coculture while attenuated by Treg coculture. Data shown are representative of five independent experiments. (B) Western blotting showed Treg coculture activated caspase-3 pathway in HIV-1/VSV infection BMM. Data shown are representative of five independent experiments. (C) Caspase-1 activation was measured by FLICA assay plus immunofluorescence microscopy. White scale bars represent 50 μm. Caspase-3 and 7 activation was measured by FLICA assay plus immunofluorescence microscopy. White scale bars represent 50 μm. (D) Proposed mechanisms Treg and Tcon use to modulate HIV-1-infected macrophage functions. Upon infection, HIV-1 activates NF-κB and the inflammasome, which results in IL-1β release. Tcon worsened this response by secretion of proinflammatory cytokines. In addition, those proinflammatory cytokines increase mitochondrial activity, which could lead to energy exhaustion and pyroptosis. However, Treg use their anti-inflammatory cytokines to extinguish the inflammation resulting from virus infection. Treg induce infected BMM apoptosis. Compared with apoptosis, pyroptosis causes more ATP release. Excessive ATP is an important inflammatory molecule and could be hydrolyzed by ecto-nucleoside triphosphate diphosphohydrolase CD39 and CD73 expressed on Treg. Moreover, Treg could transport cAMP to infected BMM and contribute to inflammation resolution. The activation status of infected macrophage affects Th0 cell differentiation. For example, M1 induce Th1 and Th17, while M2 induce Th2 and iTreg. (E) Comparison of CD39 expression on Tcon and Treg was determined by flow cytometric analysis.