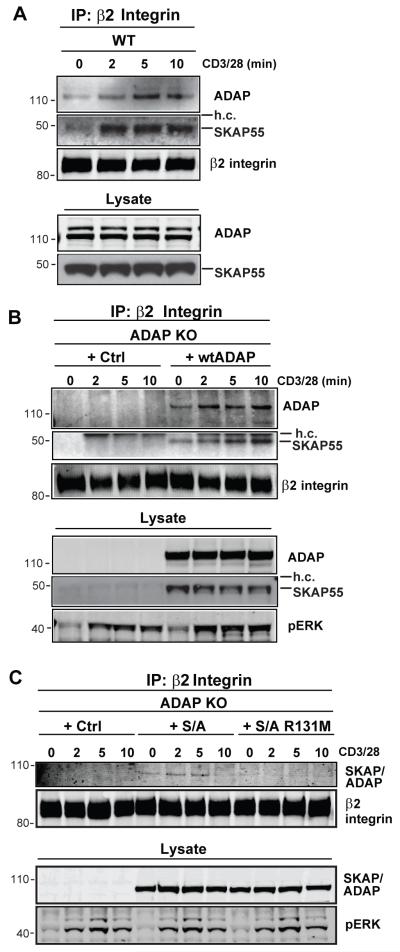
Figure 4. The SKAP55 PH domain is required for TCR-dependent recruitment to β2 integrins.
(A) hCAR/DO11.10 (WT) CD4 T cell blasts were stimulated for the indicated times with anti-CD3/CD28. The cells were lysed and subjected to anti-β2 (CD18) integrin immunoprecipitation. Western blots were probed for ADAP, SKAP55 and for the β2 integrin. Whole cell lysate inputs (lower 2 panels) are shown to demonstrate ADAP and SKAP55 expression. (B,C) hCAR/DO11.10/ADAP−/− (ADAP KO) T cell blasts were transduced with the indicated adenovirus constructs and stimulated and immunoprecipitated for β2 integrin as in (A). Western blots were probed with antibodies against ADAP and SKAP55 to detect ADAP and SKAP55 (B) or the SKAP/ADAP chimera (C), and with antibodies against the β2 integrin to detect the immunoprecipitated CD18 integrin subunit. Whole cell lysate inputs (lower panels) are shown to demonstrate expression of ADAP, SKAP55, and the SKAP/ADAP chimera, and activation was confirmed by blotting for phosphoERK. Similar results were observed in 4 independent experiments.