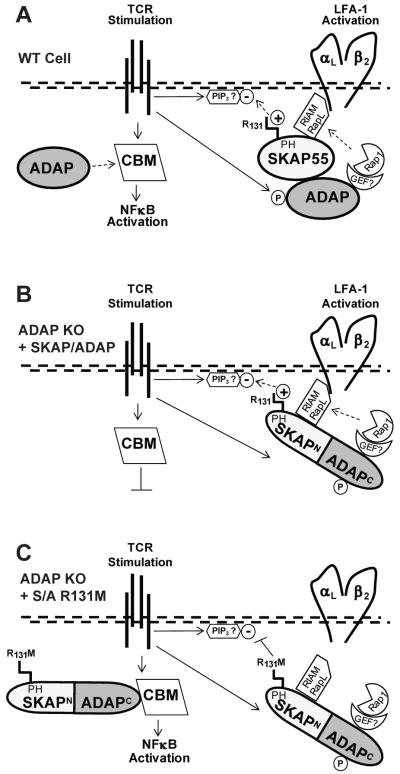
Figure 7. Model for ADAP and SKAP55 control of LFA-1 integrin activation and NF-κB activation.
(A) In wild-type T cells, TCR stimulation promotes ADAP phosphorylation (P) and activation of the constitutively associated ADAP:SKAP55 adapter module. Positively charged arginine 131 (R131) in the SKAP55 pleckstrin homology (PH) domain recruits and restricts the complex to negatively charged phosphoinositides (such as PIP3) in the plasma membrane. Downstream integrin activation components including Rap1 and a putative Rap1-GEF are provided through ADAP and the Rap1 effectors RIAM and/or RapL through SKAP55 are recruited to LFA-1 for promotion of integrin activation. Only free excess ADAP is available for concurrent assembly of the CARMA-1/Bcl10/Malt1 (CBM) complex, which initiates IκB phosphorylation/degradation and release of NFκB to the nucleus. (B) The SKAP/ADAP chimera is exclusively recruited to the integrin pathway. As there is no excess ADAP that is not associated with SKAP55 in ADAP−/− T cells expressing the chimera, no free ADAP is available for NF-κB activation. (C) In ADAP−/− cells expressing the R131M mutation in the SKAP/ADAP chimera, SKAP55 PH domain-mediated interaction of the chimera with membrane lipids is attenuated. This allows the chimera (through the ADAP C-terminus) to engage the CBM complex and promote NF-κB activation.