Abstract
Differentiation and maturation of dentate gyrus granule cells requires coordinated interactions of numerous processes. These must be regulated by protein factors capable of integrating signals mediated through diverse signalling pathways. Such integrators of inter and intracellular physiological stimuli include the cAMP-response element binding protein (CREB), a leucine-zipper class transcription factor that is activated through phosphorylation. Neuronal activity and neurotrophic factors, known to be involved in granule cell differentiation, are major physiologic regulators of CREB function. To examine whether CREB may play a role in governing coordinated gene transcription during granule cell differentiation, we determined the spatial and temporal profiles of phosphorylated (activated) CREB throughout postnatal development in immature rat hippocampus. We demonstrate that CREB activation is confined to discrete, early stages of granule cell differentiation. In addition, CREB phosphorylation occurs prior to expression of the neurotrophins BDNF and NT-3. These data indicate that in a signal transduction cascade connecting CREB and neurotrophins in the process of granule cell maturation, CREB is located upstream of neurotrophins. Importantly, CREB may be a critical component of the machinery regulating the coordinated transcription of genes contributing to the differentiation of granule cells and their integration into the dentate gyrus network.
Keywords: development, hippocampus, neurogenesis, rat, transcription factor
Introduction
In most regions of mammalian brain, production of neurons is largely confined to discrete developmental periods, and the differentiation of precursors to postmitotic neurons is completed by birth. However, in the dentate gyrus (DG) of the hippocampal formation, neuronal stem cells located at the inner border of the granule cell layer (the subgranular zone) continue to produce new granule cells (GCs) throughout adult life. This special quality of DG (Altman & Das, 1965; Kaplan & Hinds, 1977; Bayer et al., 1982), has attracted considerable interest because of its potential implications for brain repair. This attention has been further kindled by the discovery of ongoing production of new GCs, not only in rodent, but also in adult primate (Gould et al., 1999b) and human hippocampus (Eriksson et al., 1998). However, while the understanding of factors influencing the proliferation rate of this neural stem cell population has been evolving (Cameron & Gould, 1994; Cameron et al., 1995; Gould et al., 1999a; Van Praag et al., 1999), the processes that regulate differentiation and maturation of newly produced GCs remain elusive.
In the developing rat hippocampus, production of GCs begins on embryonic day 17 (E17), with proliferation of putative stem cells in a primary dentate neuroepithelium adjacent to the alveus (Altman & Bayer, 1990a). Subsequently, stem cells leave the primary dentate matrix and proceed towards the developing DG, where they form secondary and tertiary proliferation hilar matrices that give rise to the majority of GCs. Some of these stem cells eventually settle in the subgranular zone and retain their proliferative capacity (Altman & Bayer, 1990b). During development, new GCs migrate from the hilus to the GC layer, where they join its deepest portion (Schlessinger et al., 1975; Crespo et al., 1986; Rickmann et al., 1987). GC differentiation requires the interplay of multiple factors. For example, neurotrophic factors have been shown to influence the process (Lowenstein & Arsenault, 1996), and afferent innervation and neuronal activity are essential for GC dendrites to assume their mature morphology (Zafirov et al., 1994; Drakew et al., 1999). Thus, both inter and intracellular communication is required to activate and coordinate the GC differentiation program.
Cues derived from several intracellular signal transduction pathways may be integrated by transcription factors, including the cAMP-response element binding protein (CREB). CREB is a member of a transcription factor family, belonging to the basic-domain leucine-zipper class, that regulates transcription via a specific DNA target, the cAMP-response element (CRE; Brindle & Montminy, 1992; Sassone-Corsi, 1995). CREB activity requires phosphorylation at Ser-133 in response to diverse physiological signals (Gonzalez & Montminy, 1989). Once phosphorylated, CREB interacts with coactivators to govern the transcriptional machinery (Montminy, 1997). Importantly, neuronal activity (Ghosh & Greenberg, 1995; Bito et al., 1996; Deisseroth et al., 1996; Impey et al., 1996) and neurotrophic factors (Ginty et al., 1994; Finkbeiner et al., 1997) are major physiologic regulators of CREB function, and CREB activation has been suggested to contribute to GC survival (Walton & Dragunow 2000). These facts led to the supposition that CREB may play a key role in governing coordinated gene transcription during GC differentiation. To examine this hypothesis, we determined the temporal and spatial activation of CREB during postnatal development and its relationship to the expression of markers of discrete GC differentiation stages and of neurotrophins.
Materials and methods
Animals and tissue handling
Offspring of timed-pregnant Sprague-Dawley dams (Zivic-Miller, Zelienople, PA, USA) were used for these experiments. All experiments aimed to minimize pain or discomfort, were approved by the UCI Animal Care Committee and conformed to NIH guidelines. Animals were deeply anaesthesized with sodium pentobarbital and perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate buffer (PB) on postnatal days (P) 2, 5, 7, 9, 10, 12, 18, 20 or 35 (n = 4 per timepoint). Brains were removed, postfixed in perfusion buffer (4 h), cryoprotected, and frozen in −50 °C isopentane. Coronal sections (30 or 50 μm) were cut using a cryostat and collected in 0.1 M Tris-buffer for immunocytochemistry or diethylpyrocarbonate-treated 2 × SSC (0.3 M sodium chloride, 0.03 M sodium citrate) for in situ hybridization.
Immunocytochemistry
Sections were washed (2 × 5 min) in 0.1 M Tris-buffer and preincubated in the same buffer supplemented with 3% normal goat serum and 0.25% Triton X-100 (Sigma, St Louis, MO; 1 h) before being incubated with primary antisera recognizing the Ser-133-phosphorylated isoform of CREB (pCREB; rabbit polyclonal IgG, Upstate Biotechnology, Lake Placid, NY; 1: 2000) at room temperature (RT; 12 h). Sections were washed (3 × 15 min) and exposed to a secondary antiserum (biotinylated antirabbit IgG; Vectastain ABC-Kit, Vector Laboratories, Burlingame, CA; 1: 250). Antibody binding was visualized by the avidin–biotin–peroxidase reaction with 3, 3′-diaminobenzidine (DAB; Sigma, 0.05%) and hydrogen peroxide (0.002%). Control sections, incubated without the primary antibody, had no immunostaining. For detection of calbindin-D28k (rabbit polyclonal IgG, Chemicon, Temecula, CA; 1: 1000), or polysialylated neural cell adhesion molecule (PSA-NCAM; mouse monoclonal IgG, courtesy Dr Tatsunori Seki; 1: 500), Triton was excluded from the solutions. Some labelled sections were counter-stained with cresyl violet, and all were mounted, dehydrated, cleared and cover-slipped (Permount, Fisher, Pittsburgh, PA). For colocalization purposes, sections were first exposed to the pCREB-antiserum, but signal was visualized using DAB supplemented with 0.01% CoCl2 and 0.01% NiCl2. This produced a black reaction product in pCREB-immunopositive nuclei. After washing, sections were incubated with anti-PSA-NCAM or parvalbumin (monoclonal IgG, Chemicon; 1: 7000). This second immunoreaction was visualized using DAB alone, resulting in a light brown membranous (PSA-NCAM) or cytoplasmic (parvalbumin) reaction product.
BrdU labelling
Rats were injected with 5-bromo-2′-deoxyuridine (BrdU, Roche, Indianapolis, IN; 50 μg/g body weight, i.p.) on P8 and perfused 48 h later. After calbindin- or pCREB-immunocytochemistry, sections were transferred to 2x SSC, washed (2 × 15 min), immersed in 50% formamide/2x SSC to denature DNA (2 h; 65 °C) and incubated in 2 M HCl (30 min at 37 °C). Following neutralization with 0.1 M sodium borate (10 min at RT), sections were incubated with an antibody directed against BrdU (rat monoclonal IgG, Accurate Chemical, Westbury, NY, 1: 400), followed by biotinylated second antibody and the avidin–biotin–peroxidase reaction components. Benzidine dihydrochloride (Sigma, 0.01%), a peroxidase-substrate producing a blue granular staining, was used as chromogen for BrdU-detection, to permit distinction of overlapping nuclear BrdU- and pCREB-labelling.
In situ hybridization
Tissue sections (25 μm) were processed for in situ hybridization either slide-mounted (P2, P9) or free-floating (P20) as described in detail previously (Lauterborn et al., 1994). Briefly, 35S-labelled cRNA-probe for brain-derived neurotrophic factor (BDNF) exon V was transcribed (Isackson et al., 1991), yielding a 540 base length probe. For neurotrophin-3 (NT-3) mRNA localization, a 550 base 35S-cRNA was transcribed from PvuII-digested recombinant plasmid pRNT3–1 (Gall et al., 1992). Following proteinase K treatment (for P20 brain only, Sigma; 1 μg/mL), sections were hybridized for 30– 40 h at 60 °C as described (Lauterborn et al., 1994). Sections were washed, RNAse treated, and washed to a final stringency of 0.1x SSC at 60 °C for 1 h, then mounted and dipped in emulsion (NTB-2, Kodak, Rochester, NY; 1: 1 with H2O) for 4–6 weeks at 4 °C.
Results
Time course of CREB activation in the hippocampal formation
Immunostaining for pCREB resulted in the expected nuclear localization of the activated transcription factor (Ginty et al., 1993) and revealed a highly reproducible spatial and temporal pattern of pCREB-immunoreactivity in the postnatal hippocampus. During the first postnatal week, the pyramidal cell layer was well labelled (Fig. 1A). In DG, scattered hilar cells were pCREB-immunoreactive, whereas little signal was observed over the GC layer. This pattern was quite different during the second postnatal week, when pCREB-immunoreactivity was drastically reduced in the pyramidal cell layer while the GC layer labelling was increased (Fig 1B and C). However, immunoreactive cells were not equally distributed throughout the GC layer: nuclei located in the deeper (hilar) half of the layer were strongly immunoreactive for pCREB, but immunoreactivity decreased with increasing distance from the hilus, so that GCs in the superficial half were virtually unlabeled (Figs 1C and 2A). Dense pCREB-immunoreactivity was still observed during the third post-natal week, but the proportion of pCREB-immunoreactive cells was now lower, occupying only the innermost cells of the GC layer (Figs 1D and 2C). By P35, pCREB-immunoreactivity was restricted to scattered cells at the base of the GC layer (Figs 1E and F, and 3C and D) possessing irregular shaped, elongated or ellipsoid nuclei characteristic of immature granule cells (Seki & Arai, 1993).
Fig. 1.
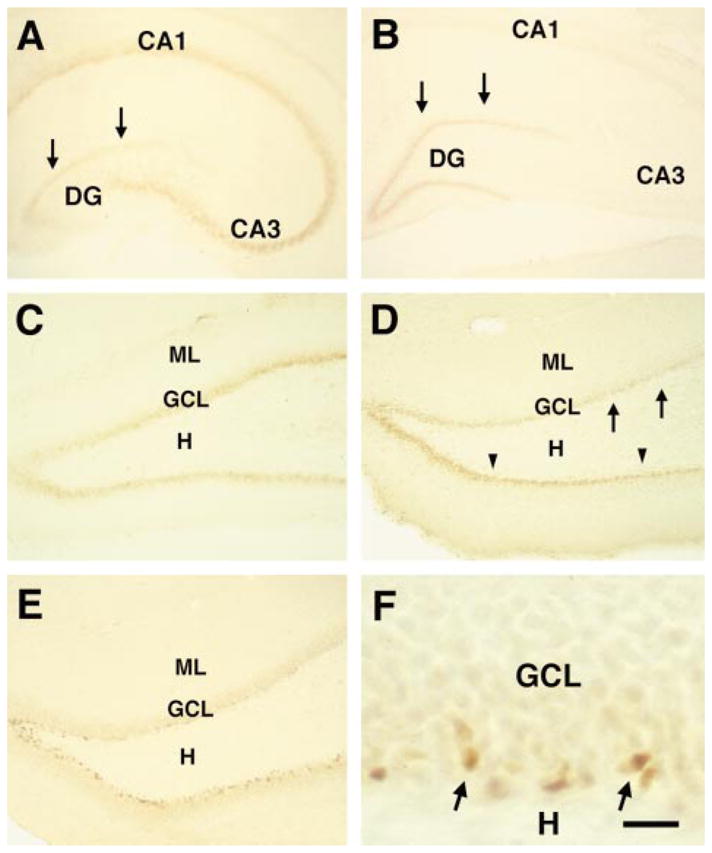
Activation pattern of CREB during postnatal hippocampal development. (A) In the hippocampal formation of the five day old rat (P5), phosphorylated CREB (pCREB) is clearly evident in the pyramidal layer (CA1 and CA3) compared with the granule cell (GC) layer (arrows). (B) By P12, hippocampal CREB activation pattern is radically altered; pCREB-immunoreactivity is almost undetectable in the pyramidal cell layer. In contrast, the GC layer (arrows), is now strongly immunoreactive. A higher magnification of the dentate gyrus (C) reveals that during the second postnatal week (P10) CREB is strongly phosphorylated in GCs, forming a 3–4 cells-deep, almost continuous band, along the hilar border of the GC layer. (D) By P18, pCREB-immunoreactive neurons still outline the hilar border, but in the earlier-maturing, lateral portion of the suprapyramidal blade (arrows), the density of immunoreactive cells is already diminished, whereas the hilar border of the infrapyramidal blade (arrowheads) remains intensely stained throughtout its length. (E) By P35, pCREB-immunoreactive neurons are scattered in a discontinuous, thin band at the GC layer hilar border. (F) a higher magnification of (E), shows variable, elongated or oval pCREB-labelled nuclei (typical of immature GCs), confined to the hilar border of the GC layer (arrows). CA, cornu ammonis; DG, dentate gyrus; GCL, granule cell layer; H, hilus; ML, molecular layer. Scale bar, 250 μm (A and B), 150 μm (C, D and E); 20 μm (F).
Fig. 2.
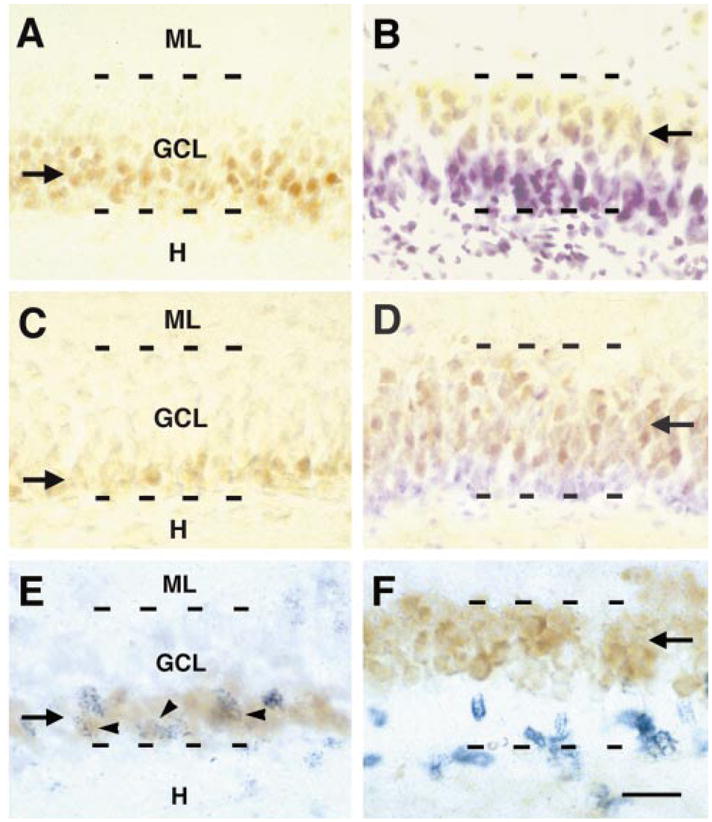
Distribution of phosphorylated CREB (pCREB), and its relationship to markers of newly formed and differentiated granule cells (GCs). Panels A–D demonstrate the granule cell layer (GCL; suprapyramidal blade) of a P10 (A and B), and of a P18 rat (C and D). Antisera against pCREB (A and C) and calbindin-D28k (B and D), a marker of differentiated GCs, reveal a virtually inverse labelling pattern, reflecting GCL maturation during the second and third postnatal weeks. The zone occupied by pCREB-immunoreactive neurons (brown reaction product; arrows in A and C) decreases progressively, whereas the zone occupied by calbindin-immunoreactive neurons increases with age (yellowish reaction product, arrows in B and D). Note that B and D are counter-stained with cresyl violet. (E and F) Immature GCs, labelled with 5-bromo-2′-deoxyuridine (BrdU), coexpress pCREB, but not calbindin-D28k. Sections represent the GCL of 10-day-old rats injected with BrdU 48 h earlier and double-labelled for BrdU and either pCREB (E) or calbindin (F). (E) BrdU-positive nuclei (blue granular reaction product), are seen in the deep, hilus-adjacent zone of the GCL, that is immunoreactive for pCREB (brown reaction product, arrow); frequent colocalization of pCREB and BrdU is evident (arrowheads). (F) BrdU-labelled nuclei (in blue) are only rarely found in the superficial, calbindin-immunoreactive zone (brown reaction product, arrow in F). H, hilus; ML, molecular layer. Scale bar, 40 μm (A–D); 20 μm (E and F).
Fig. 3.
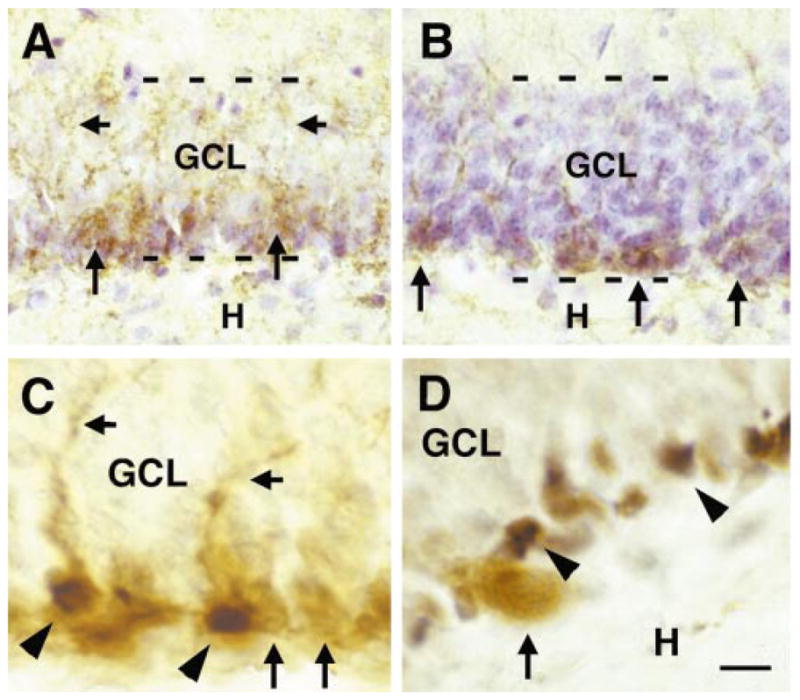
The relationship of the polysialylated neuronal cell adhesion molecule (PSA-NCAM), a marker of immature neurons, and phosphorylated CREB (pCREB) in the developing granule cell layer (GCL). (A) On P10, PSA-NCAM-immunopositive perikarya are confined to the deeper half of the GCL (arrows), and are not observed in the superficial half. The PSA-NCAM-labelled dendrites of these neurons are found in the superficial GCL zone, demonstrating that PSA-NCAM-labelled neurons already extend their dendrites towards the molecular layer (short arrows, see also C). (B) By day 35, PSA-NCAM-immunoreactive neurons are restricted to the hilar base of the GCL (arrows). (C) Double-labelling demonstrates colocalization (arrowheads) of PSA-NCAM (brown reaction product) and pCREB (black reaction product) in some but not all PSA-NCAM immunoreactive neurons. Note that neurons expressing PSA-NCAM alone are marked by arrows. (D) In contrast, double-labelling for pCREB and parvalbumin, a protein marker for interneuronal basket cells within the GCL illustrates that pCREB is selectively activated in granule cells (GC) proper. Parvalbumin-positive cells (arrow) are devoid of pCREB-immunoreactivity, whereas many surrounding GC nuclei contain pCREB (arrowheads). H, hilus. Scale bar, 20 μm (A and B); 10 μm (C and D).
Selectivity in the distribution of pCREB was evident not only in the superficial–deep axis of the GC layer, but also comparing the suprapyramidal and infrapyramidal blades. In both, pCREB was detected as early as P7. However, whereas during the third postnatal week CREB activity was clearly diminished in the suprapyramidal blade, GCs in the infrapyramidal blade were still intensely labelled (Fig. 1D). This sustained pCREB-immunoreactivity in the infrapyramidal blade may reflect a maturational delay, as both the production of GCs destined to form the infrapyramidal blade (Altman & Bayer, 1990b), and their structural differentiation (Cowan et al., 1980; Tamamaki, 1999) lags that of the suprapyramidal blade by several days.
Distribution of immature and mature granule cells in the granule cell layer
If pCREB is involved in differentiation, then its expression should be specific for late proliferative and/or early differentiation phases of immature GCs. To determine the relationship of CREB phosphorylation to proliferation of GCs, we injected rat pups (at P8) with the thymidine-analogue BrdU and perfused them 48 h later (P10). The location of newly formed, BrdU-labelled cells in the GC layer was then determined and BrdU-positive neurons were examined for pCREB-immunoreactivity. BrdU-immunopositive nuclei were abundant in the deeper, intensely pCREB-labelled zone of the GC layer. Indeed, BrdU was frequently located in pCREB-immunoreactive nuclei (Fig. 2E). BrdU-immunopositive neurons were rarely observed in the pCREB-negative, superficial zone, indicating that CREB activation in GCs is restricted to a discrete early period after their birth.
To further pinpoint the relationship of pCREB and differentiation, we immunolabeled sections with antisera directed against established markers for immature and fully differentiated GCs. The polysialylated isoform of the neural cell adhesion molecule (PSA-NCAM; Seki & Arai, 1993) was used to visualize immature GCs, whereas the calcium-binding protein calbindin-D28k (Sloviter, 1989) provided a marker for fully differentiated neurons. Patterns of PSA-NCAM- and pCREB-immunoreactivities were quite similar. Neurons expressing PSA-NCAM on their cell membrane were densely distributed throughout the deeper GC layer at P10 (Fig. 3A), but their number decreased with advancing age until, by P35, only neurons at the base of the GC layer still expressed PSA-NCAM (Fig. 3B). Double-labelling for pCREB and PSA-NCAM revealed that the two molecules were highly colocalized in these neurons (Fig. 3C), thus further supporting the notion that CREB is preferentially activated in immature GCs. The presence of PSA-NCAM-immunostaining in both GC perikarya and in dendrites that were already extending into the molecular layer illustrates that these neurons are relatively advanced in their differentiation and do not represent newborn GCs. Nuclei of parvalbumin-positive basket cells, intermingled with GCs, were mostly devoid of pCREB-immunoreactivity (Fig. 3D) further indicating the cell-type specificity and/or the differentiation-stage-specific roles for pCREB.
The pattern of calbindin-immunoreactivity was generally the inverse of those observed for pCREB and PSA-NCAM. Calbindin-expressing neurons were confined to the superficial half of the GC layer at P10 (Fig. 2B), and occupied a progressively greater proportion of the layer with increasing age (Fig. 2D). As expected from the data described above, BrdU-labelled nuclei were rarely observed in cells immunoreactive for calbindin (Fig. 2F). Together with the results of the BrdU/pCREB and PSA-NCAM/pCREB colocalization studies, these highly specific distribution patterns of pCREB and calbindin warrant the conclusion that CREB is activated in GCs early after their birth and that this activation is sustained throughout a considerable time period of their differentiation.
Expression of BDNF and NT-3 in the developing granule cell layer
Neurotrophins are a major activator of CREB function (Ginty et al., 1994; Finkbeiner et al., 1997). BDNF and NT-3 are expressed relatively early during GC layer development (Friedman et al., 1991; Dugich-Djordjevic et al., 1992; Lauterborn et al., 1994; Martinez et al., 1998), and both neurotrophins have been suggested to contribute to GC differentiation (Lowenstein & Arsenault, 1996). Previous studies on neurotrophin expression in DG, however, have mainly reported on their ontogeny and general location, but have not described in detail the location of neurotrophins within the GC layer. If CREB and neurotrophins interact to promote differentiation of GCs, their temporal and spatial expression/activation patterns should be similar. Therefore, we studied the spatial distribution of BDNF and NT-3 gene expression in DG during the first, second and third postnatal weeks using in situ hybridization. At P2, low levels of both neurotrophin mRNAs were distributed rather homogeneously throughout the barely distinct GC layer. However, by P9 and P20 both BDNF and NT3 (Fig. 4) were highly expressed in the superficial GC layer zones, but not in the deeper zones that consist of immature GCs. This expression pattern is highly consistent with the distribution of mature GCs (Fig. 2B and D). The absence of BDNF and NT-3 expression in immature GCs implies that the synthesis of neurotrophins is a late rather than an early event during GC maturation.
Fig. 4.
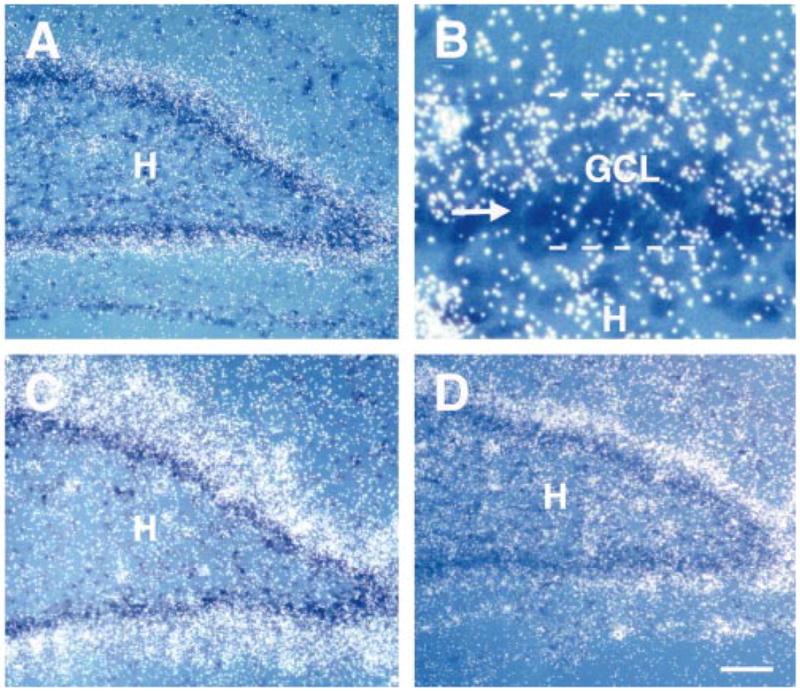
Neurotrophin expression in the dentate gyrus (DG) granule cell layer at P9 (A, B and D) and P20 (C). Sections were hybridized with 35S-labelled cRNA-probes directed against BDNF (A, B and C) or NT-3 mRNA (D), and counter-stained with cresyl violet to outline the granule cell layer (GCL). (A–C) At P9 (A and B) and P20 (C) silver grain density, a measure of BDNF mRNA abundance, was greater in the superficial (mature) zones of the GCL compared with deeper (immature) zones (arrow in B). The width of the neurotrophin-expressing, mature zone increased progressively with age (compare A and C). This gradient parallels that of GC maturation (Fig. 2). (D) NT-3 mRNA showed a similar pattern of expression to that of BDNF mRNA. H, hilus. Scale bar, 100 μm (A, C and D); 20 μm (B).
Discussion
The major results of this study are: (i) The transcription factor CREB is highly phosphorylated (activated) in the dentate gyrus GC layer during a discrete developmental period. (ii) Precise timing of CREB phosphorylation, using morphological and spatial criteria as well as proliferation and differentiation markers, indicates that CREB activation is confined to GCs during a late proliferative/early differentiation stage. (iii) Maximal CREB phosphorylation precedes neurotrophin expression in the differentiating GC layer. Therefore, we suggest that CREB may play a critical and specific role, governing transcription of genes involved in the GC differentiation program.
What specific role could CREB fulfill? Potentially, CREB function during GC differentiation may differ from its roles in adult brain. In this scenario, CREB could govern a GC-specific differentiation program by inducing the transcription of an array of stage-specific genes. Alternatively, the function of CREB in developing GCs may be closely related to its proposed role in adult networks. There is considerable evidence that CREB is a crucial player in mediating synaptic plasticity (Martin & Kandel, 1996; Silva et al., 1998). As the maturation of a newly formed neuron involves its integration into the surrounding network, a process requiring the formation and honing of neuronal connections, CREB may contribute to functional and morphological synaptogenesis in differentiating GCs.
The first alternative is supported by the demonstration that CREB, while recognizing the CRE-sequence almost exclusively in adult brain, interacts also with the related AP-1 element during the first three postnatal weeks. (Pennypacker et al., 1995). In binding AP-1, CREB may act as a hetero rather than homodimer, as the affinity of CREB-homodimers to AP-1 is weak (Foulkes et al., 1991; Hai & Curran, 1991). CREB has been shown to form heterodimers with related proteins (Foulkes et al., 1991; Hai & Curran, 1991) and such heterodimerization may alter its DNA-binding affinity, enabling induction of genes during development that are completely different from CREB’s target genes in adult brain.
The findings in the current study, however, favour the second alternative. GC production starts prior to birth (Altman & Bayer, 1990a), expression of calbindin-D28k, a marker of relatively advanced differentiation, is found in the superficial GC layer already shortly after birth (Goodman et al., 1993; Bender, unpublished observations). However, as shown in Fig. 1, CREB activity was minimal in the GC layer during the first postnatal week, and was strongly upregulated specifically during the second and third postnatal weeks. This time course is remarkable, as during the second and third postnatal weeks GCs undergo prominent morphological and physiological changes indicating their functional maturation, including formation of the majority of afferent synaptic connections (Crain et al., 1973; Cowan et al., 1980) and morphological maturation of GC dendrites (Cowan et al., 1980; Zafirov et al., 1994). Also during this period, GC axons establish giant synapses on CA3 pyramidal cell dendrites indicating maturation of their efferent connections (Amaral & Dent, 1981; Gaiarsa et al., 1992).
In this developing network, the function of CREB may parallel its proposed role in adult brain, including, for example, contribution to stabilization of functional synaptic contacts that are essential both for maturation and for maintenance of the hippocampal circuit. Thus, the maturation of GC dendrites, including spine formation, requires afferent neuronal activity. A mechanistic role for CREB in dendritic maturation and plasticity has been suggested (Murphy & Segal, 1997), and supported by Crino et al. (1998), demonstrating CREB mRNA transcription and CREB phosphorylation in response to external stimuli in developing dendrites. CREB may also participate in the establishment of new connections via presynaptic mechanisms: a close interplay between CREB and the cell adhesion molecule Fasciclin II (FasII) in the formation and honing of new synapses in Drosophila larvae neuromuscular junction has been demonstrated (Davis et al., 1996). Interestingly, the Fas II mammalian homologue, NCAM, and specifically its isoform PSA-NCAM, is essential for synaptic plasticity in the hippocampal formation (Muller et al., 1996). Recent findings providing an intracellular link between NCAM and CREB through the Ras–Mitogen-activated protein kinase/Extracellular-signal Regulated Kinase (MAPK/ERK) cascade (Schmid et al., 1999; Davis et al. 2000) and our demonstration of colocalization of pCREB and PSA-NCAM in immature GCs (a similar finding was reported by Young et al., 1999), further strengthen the assumption of a related functional interaction of these two molecules in the mammalian brain.
As noted, functional differentiation and maturation of DG during the second and third postnatal weeks involve a high degree of synapse formation and plasticity. During this stage, as in adult brain, CREB may be a key transducer of synapse-derived signals to the nucleus (Hatalski & Baram, 1997), thus coordinating the integration of immature GCs into the existing network. This differentiation-promoting role of CREB may not be restricted to GCs. We found that CREB is highly activated in hippocampal pyramidal neurons during the first postnatal week, but not later. Indeed, formation and maturation of pyramidal neurons (Bayer, 1980) and arrival of afferent entorhinal fibers (Supèr & Soriano, 1994) precede the corresponding processes in GCs by several days. Thus, earlier CREB activation in pyramidal cells supports its role in the integration of both cell types in the evolving hippocampal network.
How does this proposed role of activated CREB in differentiation fit with the established functions of neurotrophins? NT-3 and BDNF mRNA expression have been detected shortly after birth in GCs (Friedman et al., 1991; Dugich-Djordjevic et al., 1992; Lauterborn et al., 1994; Martinez et al., 1998) and a differentiation-promoting effect of both neurotrophins on GCs has been demonstrated (Lowenstein & Arsenault, 1996). It is well established that neurotrophins can induce CREB phosphorylation (Finkbeiner et al., 1997), and indeed, release of neurotrophins from mature GCs could phosphorylate CREB in immature ones. However, our observation that during the second and third postnatal weeks NT-3 and BDNF are preferentially expressed in more mature GCs, and the discovery that CREB can mediate a calcium (activity)-dependent trans-activation of the BDNF-gene via a CRE-element located in the promoter of BDNF-exon III (Shieh et al., 1998; Tao et al., 1998), prompt us to consider an alternative sequence of events. In a signal cascade connecting CREB and neurotrophins during GC differentiation, the neurotrophins may be located downstream rather than upstream of CREB. In this scenario, synaptic stimulation of immature GC dendrites induces neurotrophin-transcription via CREB, leading to neurotrophin-induced differentiation of the network, possibly through mechanisms reviewed previously (Marty et al., 1997; Klintsova & Greenough, 1999; McAllister et al., 1999; Schuman, 1999).
In summary, our results provide the first evidence for a critical role of CREB-mediated gene transcription in the differentiation of DG granule cells. In view of the rapid advances in studies of CREB function, and the large body of accumulated information regarding DG anatomy and physiology, the current findings should stimulate novel and rewarding research avenues into transcription factor regulation of the GC differentiation program.
Acknowledgments
The authors are thankful to Dr T. Seki for the PSA-NCAM antibody and to Dr Yuncai Chen for his excellent contribution. Authors’ research was supported by NIH NINDS NS26748 (to C.M.G.) and NS28912 and NS35439 (to T.Z.B.).
Abbreviations
- AP-1
activating protein 1
- BDNF
brain-derived neurotrophic factor
- BrdU
5-bromo-2′-deoxyuridine cAMP, cyclic adenosine 3′, 5′-monophosphate
- CRE
cAMP-response element
- CREB
cAMP-response element binding protein
- DAB
3, 3′-diaminobenzidine
- DG
dentate gyrus
- ERK
Extracellular-signal Regulated Kinase
- FasII
Fasciclin II
- GC
granule cell
- MAPK
Mitogen-activated protein kinase
- NT-3
neurotrophin-3
- P
postnatal
- pCREB
phosphorylated CREB
- PSA-NCAM
polysialylated neural cell adhesion molecule
- SSC
saline sodium citrate
References
- Altman J, Bayer SA. Mosaic organization of the hippocampal neuroepithelium and the multiple germinal sources of dentate granule cells. J Comp Neurol. 1990a;301:325–342. doi: 10.1002/cne.903010302. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990b;301:365–381. doi: 10.1002/cne.903010304. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GP. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–336. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Dent JA. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981;195:51–86. doi: 10.1002/cne.901950106. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Development of the hippocampal region in the rat. I. Neurogenesis examined with [3H] thymidine autoradiography. J Comp Neurol. 1980;190:87–114. doi: 10.1002/cne.901900107. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Yackel JW, Puri PS. Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science. 1982;216:890–892. doi: 10.1126/science.7079742. [DOI] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- Brindle PK, Montminy MR. The CREB family of transcription activators. Curr Opin Genetics Dev. 1992;2:199–204. doi: 10.1016/s0959-437x(05)80274-6. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15:4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan WM, Stanfield BB, Kishi K. The development of the dentate gyrus. Curr Top Dev Biol. 1980;15:103–157. [PubMed] [Google Scholar]
- Crain B, Cotman C, Taylor D, Lynch G. A quantitative electron microscopic study of synaptogenesis in the dentate gyrus of the rat. Brain Res. 1973;63:195–204. doi: 10.1016/0006-8993(73)90088-7. [DOI] [PubMed] [Google Scholar]
- Crespo D, Stanfield BB, Cowan WM. Evidence that late-generated granule cells do not simply replace earlier formed neurons in the rat dentate gyrus. Exp Brain Res. 1986;62:541–548. doi: 10.1007/BF00236032. [DOI] [PubMed] [Google Scholar]
- Crino P, Khodakhah K, Becker K, Ginsberg S, Hemby S, Eberwein J. Presence and phosphorylation of transcription factors in developing dendrites. Proc Natl Acad Sci USA. 1998;95:2313–2318. doi: 10.1073/pnas.95.5.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GW, Schuster CM, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. III. CREB is necessary for presynaptic functional plasticity. Neuron. 1996;17:669–679. doi: 10.1016/s0896-6273(00)80199-3. [DOI] [PubMed] [Google Scholar]
- Davis S, Vanhoutte P, Pagès C, Caboche J, Laroche S. The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci. 2000;20:4563–4572. doi: 10.1523/JNEUROSCI.20-12-04563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Bito H, Tsien RW. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- Drakew A, Frotscher M, Heimrich B. Blockade of neuronal activity alters spine maturation of dentate granule cells but not their dendritic arborization. Neuroscience. 1999;94:767–774. doi: 10.1016/s0306-4522(99)00378-4. [DOI] [PubMed] [Google Scholar]
- Dugich-Djordjevic MM, Tocco G, Willoughby DA, Najm I, Pasinetti G, Thompson RF, Baudry M, Lapchak PA, Hefti F. BDNF mRNA expression in the developing rat brain following kainic acid-induced seizure activity. Neuron. 1992;8:1127–1138. doi: 10.1016/0896-6273(92)90133-x. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nature Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- Foulkes NS, Borelli E, Sassone-Corsi P. CREM gene: use of alternative DNA-binding domains generates multiple antagonists of cAMP-induced transcription. Cell. 1991;64:739–749. doi: 10.1016/0092-8674(91)90503-q. [DOI] [PubMed] [Google Scholar]
- Friedman W, Ernfors P, Persson H. Transient and persistent expression of NT-3/HDNF mRNA in the rat brain during postnatal development. J Neurosci. 1991;11:1577–1584. doi: 10.1523/JNEUROSCI.11-06-01577.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiarsa JL, Beaudoin M, Ben-Ari Y. Effect of neonatal degranulation on the morphological development of rat CA3 pyramidal neurons: inductive role of mossy fibers on the formation of thorny excrescences. J Comp Neurol. 1992;321:612–625. doi: 10.1002/cne.903210408. [DOI] [PubMed] [Google Scholar]
- Gall CM, Gold SJ, Isackson PJ, Seroogy KB. Brain-derived neurotrophic factor and neurotrophin-3 mRNAs are expressed in ventral midbrain regions containing dopaminergic neurons. Mol Cell Neurosci. 1992;3:56–63. doi: 10.1016/1044-7431(92)90009-q. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Greenberg ME. Calcium signaling in neurons: molecular mechanism and cellular consequences. Science. 1995;268:239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- Ginty DD, Bonni A, Greenberg ME. Nerve growth factor activates a Ras-dependent protein kinase that stimulates c-fos transcription via phosphorylation of CREB. Cell. 1994;77:713–725. doi: 10.1016/0092-8674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Ginty DD, Kornhauser JM, Thompson MA, Bading H, Mayo KE, Takahashi JS, Greenberg ME. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science. 1993;260:238–241. doi: 10.1126/science.8097062. [DOI] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Goodman JH, Wasterlain CG, Massarweh WF, Dean E, Sollas AL, Sloviter RS. Calbindin-D28k immunoreactivity and selective vulnerability to ischemia in the dentate gyrus of the developing rat. Brain Res. 1993;606:309–314. doi: 10.1016/0006-8993(93)90999-4. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves AJ, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nature Neurosci. 1999a;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult old world primates. Proc Natl Acad Sci USA. 1999b;96:5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai T, Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci USA. 1991;88:3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatalski CG, Baram TZ. Stress-induced transcriptional regulation in the developing rat brain involves increased cyclic adenosine 3′, 5′-monophosphate-regulatory element binding activity. Mol Endocrinol. 1997;11:2016–2024. doi: 10.1210/mend.11.13.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impey S, Mark M, Villacres EC, Poser S, Chavkin C, Storm DR. Induction of CRE-mediated gene expression by stimuli that generate long-lasting LTP in area CA1 of the hippocampus. Neuron. 1996;16:973–982. doi: 10.1016/s0896-6273(00)80120-8. [DOI] [PubMed] [Google Scholar]
- Isackson PJ, Murray K, Huntsman M, Gall CM. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: temporal patterns of induction distinct from NGF. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Klintsova AY, Greenough WT. Synaptic plasticity in cortical systems. Curr Opin Neurobiol. 1999;9:203–208. doi: 10.1016/s0959-4388(99)80028-2. [DOI] [PubMed] [Google Scholar]
- Lauterborn JC, Isackson PJ, Gall CM. Cellular localization of NGF and NT-3 mRNAs in postnatal rat forebrain. Mol Cell Neurosci. 1994;5:46–62. doi: 10.1006/mcne.1994.1005. [DOI] [PubMed] [Google Scholar]
- Lowenstein DH, Arsenault L. The effects of growth factors on the survival and differentiation of cultured dentate gyrus neurons. J Neurosci. 1996;16:1759–1769. doi: 10.1523/JNEUROSCI.16-05-01759.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KC, Kandel ER. Cell adhesion molecules, CREB, and the formation of new synaptic connections. Neuron. 1996;17:567–570. doi: 10.1016/s0896-6273(00)80188-9. [DOI] [PubMed] [Google Scholar]
- Martinez A, Alcántara S, Borrell V, Del Rio JA, Blasi J, Otal R, Campo SN, Boronat A, Barbacid M, Silos-Santiago I, Soriano E. TrkB and TrkC signaling are required for maturation and synaptogenesis of hippocampal connections. J Neurosci. 1998;18:7336–7350. doi: 10.1523/JNEUROSCI.18-18-07336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty S, da Penha Berzaghi M, Berninger B. Neurotrophins and activity-dependent plasticity of cortical interneurons. Trends Neurosci. 1997;20:198–202. doi: 10.1016/s0166-2236(96)01026-0. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- Montminy M. Transcriptional regulation by cyclic AMP. Annu Rev Biochem. 1997;66:807–822. doi: 10.1146/annurev.biochem.66.1.807. [DOI] [PubMed] [Google Scholar]
- Muller D, Wang C, Skibo G, Toni N, Cremer H, Calaora V, Rougon G, Kiss JZ. PSA-NCAM is required for activity-induced synaptic plasticity. Neuron. 1996;17:413–422. doi: 10.1016/s0896-6273(00)80174-9. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Segal M. Morphological plasticity of dendritic spines in central neurons is mediated by activation of cAMP response element binding protein. Proc Natl Acad Sci USA. 1997;94:1482–1487. doi: 10.1073/pnas.94.4.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennypacker KR, Hudson PM, Hong JS, McMillian MK. DNA binding activity of CREB transcription factors during ontogeny of the central nervous system. Dev Brain Res. 1995;86:242–249. doi: 10.1016/0165-3806(95)00033-a. [DOI] [PubMed] [Google Scholar]
- Rickmann M, Amaral DG, Cowan WM. Organization of radial glial cells during the development of the rat dentate gyrus. J Comp Neurol. 1987;264:449–479. doi: 10.1002/cne.902640403. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P. Transcription factors responsive to cAMP. Annu Rev Cell Dev Biol. 1995;11:355–377. doi: 10.1146/annurev.cb.11.110195.002035. [DOI] [PubMed] [Google Scholar]
- Schlessinger AR, Cowan WM, Gottlieb DI. An autoradiographic study of the time of origin and the pattern of granule cell migration in the dentate gyrus of the rat. J Comp Neurol. 1975;159:149–176. doi: 10.1002/cne.901590202. [DOI] [PubMed] [Google Scholar]
- Schmid RS, Graff RD, Schaller MD, Chen S, Schachner M, Hemperly JJ, Maness PF. NCAM stimulates the Ras-MAPK pathway and CREB phosphorylation in neuronal cells. J Neurobiol. 1999;38:542–558. [PubMed] [Google Scholar]
- Schuman EM. Neurotrophin regulation of synaptic transmission. Curr Opin Neurobiol. 1999;9:105–109. doi: 10.1016/s0959-4388(99)80013-0. [DOI] [PubMed] [Google Scholar]
- Seki T, Arai Y. Highly polysialylated neural cell adhesion molecule (NCAM-H) is expressed by newly generated granule cells in the dentate gyrus of the adult rat. J Neurosci. 1993;13:2351–2358. doi: 10.1523/JNEUROSCI.13-06-02351.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh PB, Hu SC, Bobb K, Timmusk T, Ghosh A. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron. 1998;20:727–740. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Calcium-binding protein (Calbindin-D28k) and parvalbumin immunocytochemistry: localization in the rat hippocampus with specific reference to the selective vulnerability of hippocampal neurons to seizure activity. J Comp Neurol. 1989;280:183–196. doi: 10.1002/cne.902800203. [DOI] [PubMed] [Google Scholar]
- Supèr H, Soriano E. The organization of the embryonic and early postnatal murine hippocampus. II. Development of entorhinal, commissural, and septal connections studied with the lipophilic tracer DiI. J Comp Neurol. 1994;344:101–120. doi: 10.1002/cne.903440108. [DOI] [PubMed] [Google Scholar]
- Tamamaki N. Development of afferent fiber lamination in the infrapyramidal blade of the rat dentate gyrus. J Comp Neurol. 1999;411:257–266. [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+-influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Walton MR, Dragunow I. Is CREB a key to neuronal survival? Trends Neurosci. 2000;23:48–53. doi: 10.1016/s0166-2236(99)01500-3. [DOI] [PubMed] [Google Scholar]
- Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nature Med. 1999;5:448–453. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- Zafirov S, Heimrich B, Frotscher M. Dendritic development of dentate granule cells in the absence of their specific extrinsic afferents. J Comp Neurol. 1994;345:472–480. doi: 10.1002/cne.903450312. [DOI] [PubMed] [Google Scholar]


