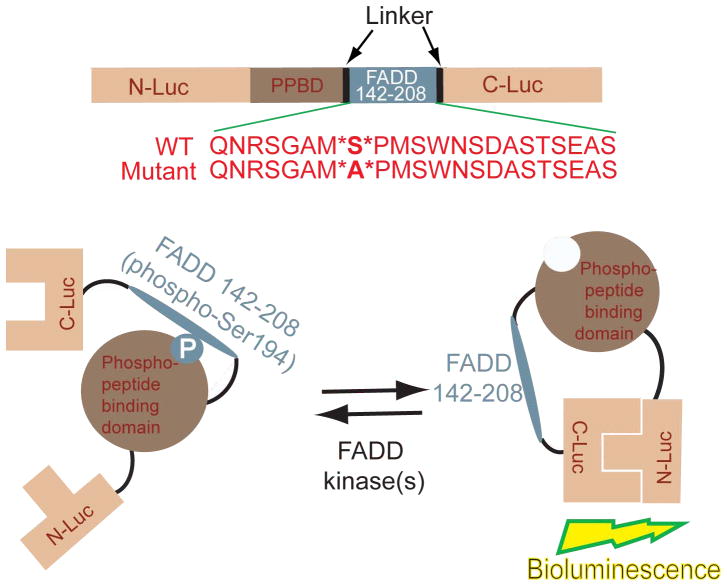
FIG. 1.
Construction of the FADD Kinase Reporter (FKR). The model shows a diagrammatic representation of the domain structure of FKR and the sequence of the C terminal domain which harbors serine 194, the target for FADD kinases. N-Luc and C-Luc are the N- and C-terminal domains of firefly luciferase that are fused to the FHA2 appropriate ends of the reporter. Two versions of the FKR were developed: the FKR (WT), which contains the wild type FADD peptide sequence and the FKR (Mutant), which contains a serine 194 substitution to alanine at the primary phosphorylation site. Phosphorylation of the FADD peptide at serine 194 results in interaction with FHA2 phosphopeptide binding domain (PPBD) causing steric constraints on C-luc and N-Luc. Inhibition of FADD kinase results in decreased binding of phospho-peptide and PPBD enabling N-Luc and C-Luc interaction to restore enzymatic activity.