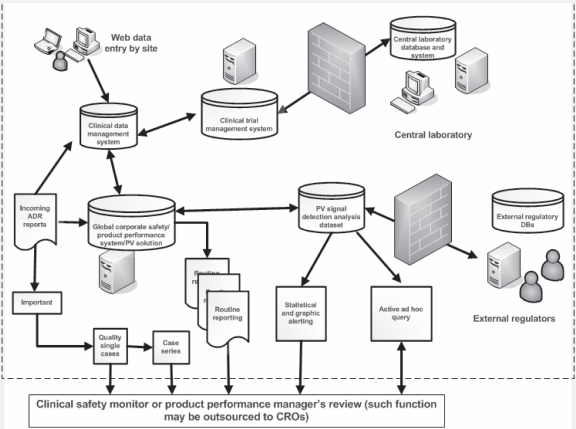
Figure 1.
The good pharmacovigilance processes and workflow in a typical sponsor biopharmaceutical firm supported by information technology. Modern internet and communication technologies have enabled improved clinical safety monitoring in a significant way despite anticipated ongoing challenges.
Abbreviations: ADR, adverse drug reaction; DB, database; PV, pharmacovigilance; CRO, clinical research organization.