Abstract
Rosuvastatin represents the latest inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase introduced in clinical practice for the treatment of hypercholesterolemia. In comparative trials, across dose ranges this statin reduced low-density lipoprotein (LDL) cholesterol and total cholesterol significantly more than atorvastatin, simvastatin, and pravastatin, and triglycerides significantly more than simvastatin and pravastatin. In healthy subjects with normal LDL cholesterol and elevated C-reactive protein, rosuvastatin treatment significantly decreased the incidence of cardiovascular events. Its chemical and pharmacokinetic properties (with a low lipophilicity and poor capacity to inhibit cytochrome P450 enzymes) suggest a very limited penetration in extrahepatic tissues with a lower risk of muscle toxicity and unlike metabolically mediated drug–drug interactions. This article reviews the most recent data on the pharmacologic and clinical properties of rosuvastatin, in order to enable the correct use of this statin for the treatment of hypercholesterolemia.
Keywords: statin, HMG-CoA reductase, LDL cholesterol, pharmacokinetics, safety
Introduction
In July 2004, an update of treatment guidelines for hypercholesterolemia was published by the National Cholesterol Education Program Adult Treatment Panel (NCEP-ATP III).1 This document reviews the results of the most recent clinical trials and assessed their implications for cholesterol management. Therapeutic lifestyle changes were emphasized as an essential modality in clinical management of dyslipidemia, and the benefit of cholesterol-lowering therapy was confirmed in high-risk patients. The ATP III low-density lipoprotein (LDL) cholesterol treatment goals and endpoints for theraputic lifestyle changes and drug therapy in different risk categories are summarized in Table 1.
Table 1.
ATP III LDL-cholesterol goals and cutpoints for therapeutic lifestyle changes and lipid-lowering therapy in different risk categories1
| Risk category | LDL-C goal | Initiate TLC | Consider drug therapy |
|---|---|---|---|
| High risk: CHD or CHD equivalentsa (10-year risk >20%) | <100 mg/dL (optional goal: <70 mg/dL) | ≥100 mg/dL | ≥100 mg/dL |
| Moderately high risk: ≥2 risk factorsb (10-year risk 10% to 20%) | <130 mg/dL | ≥130 mg/dL | ≥130 mg/dL |
| Moderate risk: ≥2 risk factorsb (10-year risk <10%) | <130 mg/dL | ≥130 mg/dL | ≥160 mg/dL |
| Low risk: 0–1 risk factor2 | <160 mg/dL | ≥160 mg/dL | ≥190 mg/dL |
CHD includes history of myocardial infarction, unstable angina, stable angina, coronary artery procedures, or evidence of clinically significant myocardial ischemia; CHD equivalents include clinical manifestations of noncoronary forms of atherosclerotic disease, diabetes, and 2 or more risk factors with 10-year risk for hard CHD > 20%.
Risk factors include: cigarette smoking, hypertension (blood pressure ≥140/90 mm Hg or on antihypertensive medication), low high-density lipoprotein (HDL) cholesterol (<40 mg/dL), family history of premature CHD, and age (men ≥ 45 years, women ≥ 55 years).
Abbreviations: LDL-C, low-density lipoprotein cholesterol; TLC, therapeutic lifestyle changes; CHD, coronary heart disease.
LDL cholesterol is the primary target of cholesterol-lowering therapy with agents such as 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (commonly referred to as statins). Currently six statins are approved and marketed in the United States and European countries. The clinical use of these drugs for primary or secondary prevention in patients with hypocholesterolemia has significantly reduced morbidity and mortality associated with coronary heart disease (CHD).2–7 Unfortunately, a number of these agents can reduce LDL cholesterol levels by only 30% to 35%, which may not bring many subjects to their NCEP-ATP III goals.8
Moreover, the higher-potency agents (simvastatin, lovastatin, atorvastatin) have extensive extrahepatic tissue penetration and show potential drug interactions that may have remarkable safety implications.9 Rosuvastatin, the most recently approved HMG-CoA reductase inhibitor, has demonstrated a potent lipid-lowering effect associated with a good tolerability profile and a lower risk of pharmacokinetic interactions. This statin is a hydrophilic compound with poor penetration in extrahepatic tissue, and it does not seem to exhibit significant cytochrome (CYP) P450 drug interactions, as do several of the other HMG-CoA reductase inhibitors.10
This reviews the pharmacologic and pharmacokinetic properties of rosuvastatin, comparing its efficacy and safety with other that of currently available HMG-CoA reductase inhibitors.
Pharmacology
Statins are natural, fungus resultant, mevinic acid derived (simvastatin, lovastatin, pravastatin), or synthetic, heptenoic acid derived (atorvastatin, cerivastatin, fluvastatin).11
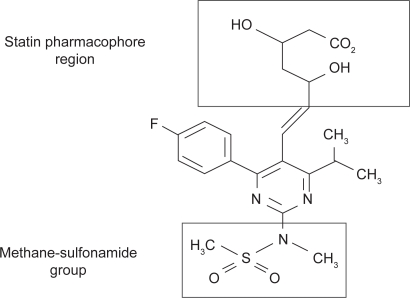
Rosuvastatin is a synthetic HMG-CoA reductase inhibitor belonging to a new novel series of methanesulfonamide pyrimidine and N-methanesulfonyl pyrrole-substituted 3,5-dihydroxy-6-heptenoates (heptenoic acid-derivative combined with a pyrimidine and sulfonamide group). The enzyme inhibition is reversible, competitive with the substrate HMG-CoA and noncompetitive with the cosubstrate nicotinamide-adenine dinucleotide phosphate (NADPH). Its low lipophilicity is conferred by the addition of a stable polar methane sulfonamide group as a hydrophilic moiety besides the characteristic statin pharmacophore.10–12
The LDL cholesterol-lowering effect of statins is derived from their inhibitory action on HMG-CoA reductase in the hepatocytes. These compounds directly bind in a tight and reversible manner to a specific binding site on the HMG-CoA reductase enzyme. Moreover, rosuvastatin and atorvastatin were found to have additional bonding interactions at the enzyme complex as compared to other statins. The addition of a methane sulfonamide group to the rosuvastatin molecule, even though it increases hydrophilicity, results in enhanced interaction with the HMG-CoA enzyme and leads to a more potent enzyme inhibition, owing to the appearance of ionic binding interactions.10–13
Rosuvastatin shows a very high affinity for the active site of the HMG-CoA reductase, four times greater than the affinity of the natural substrate HMG-CoA for the enzyme. The high affinity and tight binding of rosuvastatin to HMG-CoA reductase leads to a slow recovery of enzyme activity after removal of free inhibitor. In fact, hepatic cholesterol synthesis in rats was found to be inhibited by 62% at 7 hours after oral dosing compared to −7% and 13% for atorvastatin and simvastatin, respectively.13,14 The binding properties of this statin for the target enzyme are reflected in the drug concentration needed to block 50% of sterol synthesis in the rat hepatocyte (IC50). The mean IC50 of rosuvastatin in primary rat hepatocytes (0.16 nM on log scale) is significantly lower than the mean IC50 of other statins (1.16 for atorvastatin, 2.74 for simvastatin, 3.78 for fluvastatin, and 6.93 for pravastatin). Therefore rosuvastatin was found to be a significantly more potent inhibitor of hepatocyte sterol synthesis than any of the statins currently available.14,15
Moreover, rosuvastatin is significantly less lipophilic than other HMG-CoA reductase inhibitors except pravastatin.16 Hepatocyte concentrations of drug are not influenced by the statin lipophilicity because active uptake occurs, but differences in lipophilicity of statins are related to their penetration into extrahepatic tissues. Lipophilicity and penetration in nonhepatic tissues have potential clinical implications for muscle toxicity. Cerivastatin, the most lipophilic agent of this class, shows a very great penetration in extrahepatic tissues, is the most potent inhibitor of vascular smooth muscle proliferation, and had the largest number of cases of rhabdomyolysis. On the contrary, pravastatin, the most hydrophilic statin, has a rhabdomyolysis rate which is approximately one-third that of lovastatin and does not show a dose-related increase in the risk of muscle toxicity as with lovastatin.10,16,17
Pharmacokinetics and drug–drug interactions
The oral bioavailability of rosuvastatin is approximately 20%, or rather lower than cerivastatin (which has a bioavailability of 60%), but higher than lovastatin and simvastatin, and comparable to that of pravastatin, fluvastatin, and atorvastatin. Food is known to reduce rosuvastatin’s rate of absorption by 20%, but the extent of absorption is unchanged.
The peak plasma concentration (Cmax) of 6.1 ng/mL is reached at 5 hours after a single oral dose of 20 mg. Prolonged dosing with 20 mg once daily leads to a steady state Cmax of 9.7 ng/mL, which occurs 3 hours after dosing. In pharmacokinetic trials, the Cmax and the area under the concentration time curve (AUC0–24) demonstrated an approximately linear relation throughout the dosage range of 5 to 80 mg after single and seven daily doses. Steady state tmax ranged from 3 to 5 hours, and it was longer than other currently available statins (with tmax values usually lower than 3 hours).10,18
At steady state, the mean volume of distribution for rosuvastatin is about 130 L, and it is tightly bound in a reversible manner to plasma proteins (88%), while other statins are more than 95% protein bound except pravastatin (which is approximately 50% bound).11
Metabolism of rosuvastatin and its interactions with cytochrome P450 isoenzymes were evaluated in vitro using human hepatic microsomes. No significant inhibitory effect was noted on CYP1 A2, 2C9, 2C19, 2D6, 2E1, and 3 A4 activity. The most potent inhibition was found against the CYP2C9, but the enzyme activity was reduced by only 10%.19 The metabolism of rosuvastatin via cultured human hepatocytes (3% to 50% over 3 days) produces an N-desmethyl-metabolite and a 5S-lactone product. The N-desmethyl-metabolite is 7-fold less potent than rosuvastatin for inhibition of HMG-CoA reductase activity.10 The slow metabolism and the limited inhibitory effect of rosuvastatin on CYP isoenzymes suggest that it is unlikely to cause any significant pharmacokinetic interactions with other drugs metabolized by hepatic CYP isoenzymes. On the contrary, lovastatin, simvastatin, and atorvastatin are extensively metabolized by CYP3 A4, and inhibitors of this enzymatic system (such as itraconazole) were found to increase serum concentrations of these statins by several-fold, with an increased risk of muscle toxicity.19,20
Recovery of rosuvastatin is primarily via the fecal route of elimination: approximately 70% of absorbed dose is eliminated via bile secretion, and 30% via renal excretion. The circulating plasma half-life of rosuvastatin is about 20 hours.10,16
Statins are effective inhibitors of P-glycoprotein, which is cell membrane-associated and transports many drug substrates across the intestinal wall. Inhibition of P-glycoprotein may influence the pharmacokinetic and pharmacodynamic properties of drugs that are substrates of this transporter, and may lead to other important drug–drug interactions. Lovastatin, simvastatin, and atorvastatin are very potent inhibitors of P-glycoprotein in higher concentrations, and show important drug–drug interactions with digoxin, which is transported by P-glycoprotein.21,22 To date there are no data available assessing whether rosuvastatin is an inhibitor or a substrate for P-glycoprotein.
Itraconazole is a potent inhibitor of CYP3 A4 and P-glycoprotein, and is known to interact with other statins. In two randomized, double-blind, placebo-controlled trials the effect of itraconazole on the pharmacokinetics of rosuvastatin was evaluated in 26 healthy volunteers. In these studies, the coadministration of itraconazole produced only modest increases in plasma concentrations of rosuvastatin, which are unlikely to be of clinical relevance.23
Lipid-lowering therapy is frequently requested for the treatment of hypocholesterolemia associated with antiretroviral drugs in patients with human immunodeficiency virus (HIV) infection. Particularly, protease inhibitors (PI) are extensively metabolized by hepatic CYP 3A4 and concomitant use of statins could lead to significant drug–drug interactions with increased risk of drug toxicity. Because pharmacokinetic studies have demonstrated that the rosuvastatin metabolism is not dependent on CYP 3A4 isoenzyme, its use could be considered in PI-treated subjects owing to the low risk of pharmacological interactions.
In an open-label, randomized, prospective study we have evaluated over at least 12 months the efficacy and safety of rosuvastatin, pravastatin, and atorvastatin in 94 HIV-infected subjects on a stable PI-based antiretroviral therapy and presenting hypocholesterolemia. During the 12-month follow-up all administered statins showed a favorable tolerability profile, and patients’ plasma HIV viral load did not present any significant variation. Particularly, no significant increases in serum levels of creatine phosphokinase (CPK) or liver function tests were reported.24
However, two recent studies showed potential pharmacokinetic interactions between rosuvastatin and PIs.
A prospective study assessed drug–drug interactions of rosuvastatin with atazanavir/ritonavir and fosamprenavir/ritonavir in 6 HIV-negative healthy adult volunteers. Compared to baseline, AUC0–24 and Cmax of rosuvastatin increased by 213% and 600%, respectively, when given with atazanavir/ritonavir, while coadministration with fosamprenavir/ritonavir did not significantly affect plasma concentrations of the statin. Therefore, atazanavir/ritonavir significantly increases the plasma levels of rosuvastatin, most likely by increasing its oral bioavailability, but the clinical significance of this pharmacokinetic interaction is unknown.25
An open-label, prospective study evaluated drug-drug interaction between rosuvastatin and lopinavir/ritonavir in 15 HIV-negative healthy adult volunteers. Rosuvastatin AUC0–24 and Cmax were unexpectedly increased 2.1- and 4.7-fold, respectively, when given in combination with the PI, and there was 1 asymptomatic elevation in serum levels of CPK and liver function tests. Therefore, rosuvastatin and lopinavir/ritonavir should be used with caution until the safety and appropriate dosing of this combination have been demonstrated in larger populations.26
Laboratory and clinical efficacy
Rosuvastatin was found to be highly efficacious in reducing plasma LDL cholesterol levels and favorably modifying the other lipid and apolipoprotein parameters in patients with hypercholesterolemia in both short-term and long-term clinical trials. Moreover, in pairwise comparisons, rosuvastatin showed a significantly higher efficacy in reducing LDL cholesterol than milligram-equivalent doses of other statins currently available.
Dosing of rosuvastatin ranging from 1 to 80 mg daily over 6 weeks has resulted in LDL-cholesterol reductions compared with the baseline values from 34% to 65%, and the LDL cholesterol reduction at the probable starting dose range (5 to 10 mg) was 43% to 51%.27
In a direct comparative trial, rosuvastatin or atorvastatin was administered to patients with familial heterozygous hypercholesterolemia. Subjects were randomized to either 20 mg of rosuvastatin or atorvastatin and the dose was doubled at 6-week periods until all patients reached the maximum dose of 80 mg for both drugs at weeks 12 to 18. Decreases in LDL cholesterol concentrations were significantly greater for rosuvastatin compared with atorvastatin at all time points, and 61% of patients taking rosuvastatin reached target goals according to NCEP-ATP II guidelines, compared to 46% of those taking atorvastatin.28
The STELLAR study29 was a 6-week, open-label, randomized, multicenter trial comparing rosuvastatin with atorvastatin, pravastatin, and simvastatin across dose ranges for reduction of LDL cholesterol, changes in other lipid parameters, and achievement of NCEP-ATP III LDL-cholesterol goals.
After a 6-week dietary lead-in period, 2431 adult subjects with hypercholesterolemia were randomized to therapy with rosuvastatin (10, 20, 40, or 80 mg daily), atorvastatin (10, 20, 40, or 80 mg daily), simvastatin (10, 20, 40, or 80 mg daily), or pravastatin (10, 20, or 40 mg daily). At the end of 6-weeks follow-up, across-dose analyses showed that rosuvastatin 10 to 80 mg reduced LDL cholesterol by a mean of 8.2% more than atorvastatin 10 to 80 mg, 26% more than pravastatin 10 to 40 mg, and 12%–18% more than simvastatin 10 to 80 mg (all P < 0.001). Mean percent changes in high-density lipoprotein (HDL) cholesterol levels were +7.7% to +9.6% compared with +2.1% to +6.8% in all other groups. Moreover, rosuvastatin reduced total cholesterol significantly more than all comparators, and triglycerides significantly more than simvastatin and pravastatin. The NCEP-ATP III LDL cholesterol goals were achieved by 82% to 89% of subjects treated with rosuvastatin 10 to 40 mg compared with 69% to 85% of patients treated with atorvastatin 10 to 80 mg, and drug tolerability was comparable across treatments.29 Effects of statins at different dosages in lowering LDL cholesterol levels are summarized in Table 2.
Table 2.
Efficacy of statins at different daily doses in reducing LDL cholesterol concentrations versus respective baseline values after a 6-week therapy1,29
| Daily doses | 10 mg | 20 mg | 40 mg | 80 mg |
|---|---|---|---|---|
| Pravastatin | –20% | –24% | –34% | – |
| Simvastatin | –28% | –35% | –41% | –46% |
| Atorvastatin | –38% | –43% | –48% | –51% |
| Rosuvastatin | –45% | –52% | –55% | – |
A substudy of the STELLAR trial assessed non-HDL cholesterol, apolipoprotein (apo) B, and lipid and apolipoprotein ratios that included both atherogenic and antiatherogenic lipid components in 2,268 patients with hypercholesterolemia. All participants were randomized to therapy with rosuvastatin (10, 20, 40, or 80 mg daily), atorvastatin (10, 20, 40, or 80 mg daily), simvastatin (10, 20, 40, or 80 mg daily), or pravastatin (10, 20, or 40 mg daily) for 6 weeks. At the end of follow-up, rosuvastatin reduced non-HDL cholesterol, apo B, and all lipid and apolipoprotein ratios assessed significantly more than milligram-equivalent doses of atorvastatin, and milligram-equivalent or higher doses of simvastatin and pravastatin (all P < 0.002).30
A post-hoc subanalysis of the STELLAR trial evaluated the effects of maximal doses of rosuvastatin and atorvastatin on LDL cholesterol and small dense LDL (sLDL) cholesterol levels in 271 hyperlipidemic patients. All participants were randomized to therapy with rosuvastatin 40 mg daily or atorvastatin 80 mg daily for 6 weeks. Rosuvastatin was significantly more effective than atorvastatin in reducing LDL cholesterol, sLDL cholesterol, total cholesterol/HDL cholesterol ratio, and non-HDL cholesterol, even though the magnitude of these differences was modest, and both statins caused similar decreases in triglyceride levels.31
Two studies have evaluated the efficacy of rosuvastatin therapy after a 52-week follow-up. In the first randomized, double-blinded, multicenter study, 412 subjects with elevated LDL cholesterol levels received fixed doses of rosuvastatin (5 or 10 mg daily) or atorvastatin (10 mg daily) for 12 weeks, followed by dose adjustements up to 80 mg if the NCEP-ATP III goals were not met. Both doses of rosuvastatin resulted in greater LDL cholesterol reductions than atorvastatin at 12 weeks (46% and 50%, respectively, versus 39%; P < 0.001 for both groups) and 52 weeks (47% and 53%, respectively, versus 44%; P < 0.05 and P < 0.001).32
In a similarly designed trial, 477 patients with hypocholesterolemia received fixed doses of rosuvastatin (5 or 10 mg daily), pravastatin (20 mg), or simvastatin (20 mg) for 12 weeks followed by 40 weeks of liberal-dose titration up to 80 mg for rosuvastatin and simvastatin and 40 mg for pravastatin. After 52 weeks, more rosuvastatin-treated subjects achieved the NCEP-ATP III LDL cholesterol goals (88% and 87.5%, respectively) than recipients of pravastatin (60%) or simvastatin (73%).33
In recent years, some evidence has suggested that the “quality” rather than the “quantity” of LDL produces a direct influence on cardiovascular risk. There are at least four major subspecies of LDL particles, and the predominance of sLDL has been associated with a significantly higher risk of coronary artery disease.34 To date, only few studies have directly investigated if rosuvastatin may alter LDL size and subclasses, but this statin seems modulate significantly LDL size and subclasses towards less atherogenic particles as well as the LDL particle number, as indirectly measured by the levels of apo B.35
The JUPITER trial36 was a randomized, double-blind, placebo-controlled study designed to compare whether rosuvastatin (20 mg daily) versus placebo would reduce major cardiovascular events in 17,802 apparently healthy patients with low to normal LDL cholesterol levels but elevated C-reactive protein (CRP) levels. Notably, this trial was prematurely interrupted after a median follow-up of 1.9 years based on unequivocal evidence of a reduction in cardiovascular morbidity and mortality in rosuvastatin-treated patients. The rates of first major cardiovascular events (defined as non-fatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, arterial revascularization procedure, or confirmed death from cardiovascular causes) were 0.77 and 1.36 per 100 person-years of follow-up in the rosuvastatin and placebo groups, respectively (P < 0.00001).36
In an analysis of 15,548 subjects participating in the JUPITER trial (87% of full cohort), patients allocated to rosuvastatin who achieved LDL cholesterol less than 1.8 mmol/L had a 55% reduction in rate of vascular events, and those achieving CRP less than 2 mg/L a 62% reduction. Particularly, in rosuvastatin-treated patients achieving both LDL cholesterol less than 1.8 mmol/L and CRP less than 2 mg/L, a 65% reduction in vascular events was recorded.37
Safety
The HMG-CoA reductase inhibitors are overall very safe, especially in light of the ability of this drug class to reduce morbidity and mortality. However, the withdrawal of cerivastatin because of excess cases of fatal rhabdomyolysis has raised awareness of this otherwise very rare complication of treatment, and there is an appropriately enhanced concern about adverse effects associated with these drugs.
From 1997 to 2000 there were 387 cases of rhabdomyolysis associated with cerivastatin therapy, which represented about 50% of all of the cases spontaneously reported in association with statin therapy over this period. In comparison, simvastatin was responsible for 24% of the total, atorvastatin for 11%, and pravastatin for 9%. In a recent report using US data, there were 0.15 cases of death from rhabdomyolysis per million prescriptions for all statins, but 3.16 cases per million prescriptions for cerivastatin alone. The relative high number of cases of rhabdomyolysis for cerivastatin may be related to its greater lipophilicity and penetration in extrahepatic tissues, as described previously.38,39 However, a population-based cohort study found that the use of statins in general was associated with a greater risk of myopathy than nonuse, but the absolute risk of myopathy was minimal.40
A review of rosuvastatin’s pooled safety data demonstrated that incidence of adverse events was comparable to those of the other statins. The most frequently reported adverse events (incidence >5%) during rosuvastatin therapy in the controlled phase II/III trials included pharyngitis (12.2%), pain (6.7%), headache (6.6%), flu syndrome (5.3%), and myalgia (5.1%). The frequency of elevation in alanine amino-transferase (ALT) was 0.5%, and the incidence of myopathy (CPK levels > 10 times upper limit of normal (ULN) with muscle symptoms including weakness, aching, or tenderness) was 0.2%. All cases of myopathy occurred in patients treated with 80 mg daily and CPK elevations resolved following withdrawal of rosuvastatin.41
A retrospective matched cohort study compared the incidence rates of hospitalization associated with rhabdomyolysis, myopathy, renal or hepatic dysfunction, and of inhospital death, among over 48,000 initiators of rosuvastatin and other statins. This study found no difference between rosuvastatin and the other statins in the incidence of hospitalization because of renal or hepatic dysfunction, or death, while the absolute incidence rates of rhabdomyloysis and myopathy were reassuringly low among all statin initiators but remain too small for firm conclusions to be drawn on any difference between the statins. Particularly, incidence rate per 1000 person-years of rhabdomyolysis was 0.1 among subjects taking rosuvastatin and 0.06 among those taking other statins, while incidence rate of myopathy was 0.2 among rosuvastatin-treated subjects and 0 among other statin initiators.42
The safety and tolerability of rosuvastatin were evaluated using data from 16,876 patients who received rosuvastatin 5 to 40 mg daily in a multinational phase II/III/IIIb/IV program. Adverse events irrespective of causality assessment occurred in 52.1% of patients receiving rosuvastatin and 51.8% of those receiving placebo. In all controlled clinical trials with comparator statins, rosuvastatin 5 to 40 mg was associated with an adverse event profile similar to profiles for atorvastatin 10 to 80 mg, simvastatin 10 to 80 mg, and pravastatin 10 to 40 mg. Clinically significant elevations in ALT were uncommon in the rosuvastatin and comparator groups, and elevated CPK levels more than 10 times laboratory ULN occurred in ≤0.3% of patients receiving rosuvastatin or other statins. Myopathy possibly related to treatment was reported in 0.03% of patients taking rosuvastatin at doses of 5 to 40 mg. The frequency of dipstick-positive proteinuria at a rosuvastatin dose 5 to 40 mg was comparable to that seen with other statins, and the occurrence of proteinuria was not predictive of acute or chronic renal disease. However, both short- and long-term rosuvastatin treatments were associated with small increases in estimated glomerular filtration rate.43
An observational cohort study was designed to monitor the postmarketing safety of rosuvastatin using prescription-event monitoring. The cohort comprised 11,680 patients with a median period of treatment of 9.8 months, and rosuvastatin was found to be reasonably well tolerated. Myalgia was the most common reason for stopping rosuvastatin and the most frequently reported clinical event. A 2.5-fold increase in the rate of abnormal liver function tests was observed for patients started on rosuvastatin 40 mg daily compared with those started on 10 mg daily, while no case of rhabdomyolysis was reported in this cohort.44
Anti-inflammatory effects of rosuvastatin
Recent data have show multiple mechanisms for statins in reducing the risk of cardiovascular diseases. In particular, the HMG-CoA reductase inhibitors reduce the incidence of ischemic heart disease, improve endothelial function, reduce left ventricular hypertrophy and adverse remodeling, slow atherosclerotic process, and reduce serum levels of pro-inflammatory cytokines.45
Recently, the in vitro effects of statins on peripheral blood mononuclear cells and fibroblast-like synoviocytes were analyzed in 25 patients with rheumatoid arthritis and in 20 healthy blood donors. In fibroblast-like cells stimulated with atorvastatin a significant downregulation of proinflammatory cytokine (interleukin-6) and chemokine (interleukin-8) expression was detected, showing a marked in vitro anti-inflammatory activity of atorvastatin in rheumatoid arthritis, including a systemic effect on a pathogenic CD4+ T cell population and a local effect on fibroblast-like synoviocytes.46 Moreover, simvastatin and atorvastatin inhibited the CRP-induced chemokine secretion, intercellular adhesion molecule (ICAM-1) upregulation, and migration in human adherent monocytes, through the inhibition of HMG-CoA reductase-extracellular signal-regulated kinase 1/2 pathway.47
The effect of rosuvastatin on plasma inflammation markers, endogenous nitric oxide synthase inhibitor levels, and reactive oxygen species generated by circulating leukocytes was evaluated in normotensive and in spontaneously hypertensive rats. In the experimental conditions, rosuvastatin lessened pro-inflammatory cytokines, increased interleukin-4 levels, and reduced reactive oxygen species production in circulating monocytes of spontaneously hypertensive rats.48
The effect of rosuvastatin on CRP expression in stimulated human hepatocytes was also investigated. Experimental results showed a direct inhibitory effect of rosuvastatin on interleukin-6-induced expression of CRP in human hepatoma cells and primary human hepatocytes. Statins may reduce C-reactive protein levels by inhibiting its production in the liver rather than by exerting systemic anti-inflammatory effects.49
The anti-inflammatory properties of rosuvastatin and other HMG-CoA reductase inhibitors may have a great clinical utility in addition to their effects on atherogenic lipoproteins, and certainly require further laboratory and clinical in-depth examination.
Conclusions
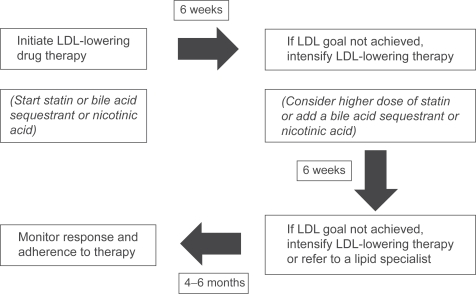
The most appropriate algorithm for pharmacologic therapy in patients with hypocholesterolemia is summarized in Figure 2.
Figure 2.
Algorithm for drug-therapy of hypercholesterolemia.
Abbreviation: LDL, low-density lipoprotein.
Rosuvastatin could be considered a second-generation HMG-CoA reductase inhibitor with unique pharmacokinetic and pharmacodynamic properties. Its chemical structure allows additional HMG-CoA reductase enzyme-binding interactions that cause tighter binding, and in vitro studies have shown a substantial active transport into hepatocytes, with the lowest IC50 for sterol synthesis in liver cells. As a consequence, rosuvastatin at dosages ranging from 10 to 80 mg daily has demonstrated superior efficacy in lowering LDL cholesterol levels compared to milligram-equivalent dosages of atorvastatin, simvastatin, and pravastatin. Moreover, the lower lipophilicity of rosuvastatin is associated with poor penetration in extrahepatic tissues such as human umbilical vein endothelial cells and fibroblasts, with a lower risk of muscle toxicity. Rosuvastatin metabolism is characterized by a low potential for cytochrome P450 involvement and a lower risk of drug–drug interactions with other drugs extensively metabolized by this hepatic pathway. This statin was usually well tolerated and the safety profile was comparable to that of other statins currently available, and has shown significant anti-inflammatory effects in experimental conditions. In this context, rosuvastatin could be very helpful for the treatment of hypercholesterolemia.
Figure 1.
Rosuvastatin chemical structure.
Footnotes
Disclosures
The author discloses no conflicts of interest.
References
- 1.Grundy SM, Cleeman JI, Merz NB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 2.Fonarow GC, French WJ, Parsons LS, et al. Use of lipid-lowering medications at discharge in patients with acute myocardial infarction. Circulation. 2001;103:38–44. doi: 10.1161/01.cir.103.1.38. [DOI] [PubMed] [Google Scholar]
- 3.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial inferction with average cholesterol levels. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 4.Scandinavian Simvastatin Survival Study Group Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 5.Downs JR, Clearfield M, Wels S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. JAMA. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz GG, Olsson AG, Ezkowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study. JAMA. 2001;285:1711–1716. doi: 10.1001/jama.285.13.1711. [DOI] [PubMed] [Google Scholar]
- 7.LIPID Study Group Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 8.Jones P, Kafonek S, Laurora I, Hunninghake D. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (the CURVES Study) Am J Cardiol. 1998;81:582–587. doi: 10.1016/s0002-9149(97)00965-x. [DOI] [PubMed] [Google Scholar]
- 9.White CM. An evaluation of CYP3A4 drug interactions with HMG CoA reductase inhibitors. Formulary. 2000;35:343–352. [Google Scholar]
- 10.White CM. A review of the pharmacologic and pharmacokinetic aspects of rosuvastatin. J Clin Pharmacol. 2002;42:963–970. [PubMed] [Google Scholar]
- 11.White CM, Chow MS. A review of HMG CoA reductase inhibitors. US Pharmacist. 1998;23:HS19–HS28. [Google Scholar]
- 12.Watanabe M, Koike H, Ishiba T, Okada T, Seo S, Hirai K. Synthesis and biological activity of methanesulfonamide pyrimidine- and N-methanesulfonyl pyrrole-substituted 3,5-dihydroxy-6 heptenoates, a novel series of MHG CoA reductase inhibitors. Bioorg Med Chem. 1997;5:437–444. doi: 10.1016/s0968-0896(96)00248-9. [DOI] [PubMed] [Google Scholar]
- 13.Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG CoA reductase. Science. 2001;292:1160–1164. doi: 10.1126/science.1059344. [DOI] [PubMed] [Google Scholar]
- 14.Smith D, Davidson R, Bloor S, et al. Pharmacological properties of ZD4522: a new HMG CoA reductase inhibitor. Atherosclerosis. 2000;151:39. [Google Scholar]
- 15.Buckett L, Ballard P, Davidson R, et al. Selectivity of ZD4522 for inhibition of cholesterol synthesis in hepatic versus non-hepatic cells. Atherosclerosis. 2000;151:41. [Google Scholar]
- 16.McTaggart F, Buckett L, Davidson R, et al. Preclinical and clinical pharmacology of rosuvastatin, a new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Am J Cardiol. 2001;87(Suppl 5 A):28B–32B. doi: 10.1016/s0002-9149(01)01454-0. [DOI] [PubMed] [Google Scholar]
- 17.Negre-Aminou P, Van Vliet AK, Van Erck M, et al. Inhibition of proliferation of human smooth muscle cells by various HMG CoA reductase inhibitors: comparison with other human cell types. Biochemica et Biophysica Acta. 1997;1345:259–268. doi: 10.1016/s0005-2760(96)00184-1. [DOI] [PubMed] [Google Scholar]
- 18.Warwick MJ, Dane AL, Raza A, Scheneck DW. Single and multiple-dose pharmacokinetics and safety of the new HMG CoA reductase inhibitor ZD4522. Atherosclerosis. 2000;151:39. [Google Scholar]
- 19.Mcormick AD, McKillop D, Butters CJ, et al. ZD4522- an HMG CoA reductase inhibitor free metabolically mediated drug interactions: metabolic studies in human in vitro systems. J Clin Pharmacol. 2000;40:1055. [Google Scholar]
- 20.Omar MA, Wilson JP, Cox TS. Rhabdomyolysis and HMG CoA reductase inhibitors. Ann Pharmacother. 2001;35:1096–1107. doi: 10.1345/aph.10228. [DOI] [PubMed] [Google Scholar]
- 21.Wang E, Casciano CN, Clement RP, Johnson WW. HMG CoA reductase inhibitors (statins) characterized as direct inhibitors of P-glycoprotein. Pharm Res. 2001;18:800–806. doi: 10.1023/a:1011036428972. [DOI] [PubMed] [Google Scholar]
- 22.Boyd RA, Stern RH, Stewart BH. Atorvastatin coadministration may increase digoxin concentrations by inhibition of intestinal P-glycoprotein-mediated secretion. J Clin Pharmacol. 2000;40:91–98. doi: 10.1177/00912700022008612. [DOI] [PubMed] [Google Scholar]
- 23.Cooper KJ, Martin PD, Dane AL, et al. Effect of itraconazole on the pharmacokinetics of rosuvastatin. Clin Pharmacol Ther. 2003;73:322–329. doi: 10.1016/s0009-9236(02)17633-8. [DOI] [PubMed] [Google Scholar]
- 24.Calza L, Manfredi R, Colangeli V, Pocaterra D, Pavoni M, Chiodo F. Rosuvastatin, pravastatin, and atorvastatin for the treatment of hypercholesterolemia in HIV-infected patients receiving protease inhibitors. Curr HIV Res. 2008;6:572–578. doi: 10.2174/157016208786501481. [DOI] [PubMed] [Google Scholar]
- 25.Busti AJ, Bain AM, Hall RG, et al. Effects of atazanavir/ritonavir or fosamprenavir/ritonavir on the pharmacokinetics of rosuvastatin. J Cardiovasc Pharmacol. 2008;51:605–610. doi: 10.1097/FJC.0b013e31817b5b5a. [DOI] [PubMed] [Google Scholar]
- 26.Kiser JJ, Gerber JG, Predhomme JA, et al. Drug-drug interaction between lopinavir/ritonavir and rosuvastatin in healthy volunteers. J Acquir Immune Defic Syndr. 2008;47:570–578. doi: 10.1097/QAI.0b013e318160a542. [DOI] [PubMed] [Google Scholar]
- 27.Olsson AG, Pears J, McKellar J, Mizan J, Raza A. Effect of rosuvastatin on low-density lipoprotein cholesterol in patients with hypercholesterolemia. Am J Cardiol. 2001;88:504–508. doi: 10.1016/s0002-9149(01)01727-1. [DOI] [PubMed] [Google Scholar]
- 28.Stein E, Strutt KL, Miller E, Southworth H. ZD4522 is superior to atorvastatin in the treatment of patients with heterozygous familial hypercholesterolemia. J Am Coll Cardiol. 2001;37(Suppl):292A. [Google Scholar]
- 29.Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR Trial) Am J Cardiol. 2003;92:152–160. doi: 10.1016/s0002-9149(03)00530-7. [DOI] [PubMed] [Google Scholar]
- 30.Jones PH, Hunninghake DB, Ferdinand KC, et al. Effects of rosuvastatin versus atorvastatin, simvastatin, and pravastatin on non-high-density lipoprotein cholesterol, apolipoproteins, and lipid ratios in patients with hypercholesterolemia: additional results from the STELLAR trial. Clin Therap. 2004;26:1388–1399. doi: 10.1016/j.clinthera.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Ai M, Otokozawa S, Asztalos BF, et al. Effects of maximal doses of atorvastatin versus rosuvastatin on small dense low-density lipoprotein cholesterol levels. Am J Cardiol. 2008;101:315–318. doi: 10.1016/j.amjcard.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 32.Olsson AG, Istad H, Luurila O, et al. Effects of rosuvastatin and atorvastatin compared over 52 weeks of treatment in patients with hypercholesterolemia. Am Heart J. 2002;144:1044–1051. doi: 10.1067/mhj.2002.128049. [DOI] [PubMed] [Google Scholar]
- 33.Brown WV, Bays HE, Hassman DR, et al. Efficacy and safety of rosuvastatin compared with pravastatin and simvastatin in patients with hypercholesterolemia: a randomized, double-blind, 52-week trial. Am Heart J. 2002;144:1036–1043. doi: 10.1067/mhj.2002.129312. [DOI] [PubMed] [Google Scholar]
- 34.Julius U, Dittrich M, Pietzsch J. Factors influencing the formation of small dense low-density lipoprotein particles in dependence on the presence of the metabolic syndrome and on the degree of glucose intolerance. Int J Clin Pract. 2007;61:1798–1804. doi: 10.1111/j.1742-1241.2007.01507.x. [DOI] [PubMed] [Google Scholar]
- 35.Rizzo M, Berneis K, Spinas GA, Rini GB, Kapur NK. Quantitative and qualitative effects of rosuvastatin on LDL-cholesterol: what is the clinical significance? Int J Clin Pract. 2009;63:478–485. doi: 10.1111/j.1742-1241.2008.01979.x. [DOI] [PubMed] [Google Scholar]
- 36.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2280–2282. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 37.Ridker PM, Danielson E, Fonseca FA, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373:1175–1182. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 38.Petition to the FDA to issue strong warnings about the potential for certain cholesterol lowering drugs to cause potentially life-threatening muscle damage. Retrieved from http:/www.citizen.org/publications/release.cfm?ID=7051.
- 39.Staffa J. Letter to the editor. N Engl J Med. 2002;346:539–540. doi: 10.1056/NEJM200202143460721. [DOI] [PubMed] [Google Scholar]
- 40.Gaist D, Rodriguez LA, Huerta C, Hallas J, Sindrup SH. Lipid-lowering drugs and risk of myopathy: a population-based follow-up study. Epidemiology. 2001;12:565–569. doi: 10.1097/00001648-200109000-00017. [DOI] [PubMed] [Google Scholar]
- 41.Shepherd J, Hunninghake D, Harris S, Hutchinson H, Pears J. A review of the safety profile of rosuvastatin in an international phase II/III clinical trial program. Paper presented at the XIV International Symposium on Drugs Affecting Lipid Metabolism; New York. September 2001. [Google Scholar]
- 42.McAfee AT, Ming EE, Seeger JD, et al. The comparative safety of rosuvastatin: a retrospective matched cohort study in over 48000 initiators of statin therapy. Pharmacoepidemiol Drug Saf. 2006;15:444–453. doi: 10.1002/pds.1281. [DOI] [PubMed] [Google Scholar]
- 43.Shepherd J, Vidt DG, Miller E, Harris S, Blasetto J. Safety of rosuvastatin: update on 16876 rosuvastatin-treated patients in a multinational clinical trial program. Cardiology. 2007;107:433–443. doi: 10.1159/000100908. [DOI] [PubMed] [Google Scholar]
- 44.Kasliwai R, Wilton LV, Cornelius V, Aurich-Barrera B, Shakir SA. Safety profile of rosuvastatin: results of a prescription-event monitoring study of 11680 patients. Drug Saf. 2007;30:157–170. doi: 10.2165/00002018-200730020-00005. [DOI] [PubMed] [Google Scholar]
- 45.Riccioni G. Statins and carotid intima-media thickness reduction: an up-do-date review. Curr Med Chem. 2009;16:1799–1805. doi: 10.2174/092986709788186183. [DOI] [PubMed] [Google Scholar]
- 46.Blaschke S, Viereck V, Schwarz G, Klinger HM, Guerluek S, Muller GA. Anti-inflammatory effects of atorvastatin on peripheral blood mononuclear cells and synovial fibroblasts in rheumatoid arthritis. Scand J Rheumatol. 2009 Feb 26;:1–5. doi: 10.1080/03009740802572475. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 47.Montecucco F, Burger F, Pelli G, et al. Statins inhibit C-reactive protein-induced chemokine secretion, ICAM-1 upregulation and chemotaxis in adherent human monocytes. Rheumatology (Oxford) 2009;48:233–242. doi: 10.1093/rheumatology/ken466. [DOI] [PubMed] [Google Scholar]
- 48.Sicard P, Delemasure S, Korandji C, et al. Anti-hypertensive effects of rosuvastatin are associated with decreased inflammation and oxidative stress markers in hypertensive rats. Free Radic Res. 2008;42:226–236. doi: 10.1080/10715760701885380. [DOI] [PubMed] [Google Scholar]
- 49.Mayer C, Gruber HJ, Landl EM, et al. Rosuvastatin reduces interleukin-6-induced expression of C-reactive protein in human hepatocytes in a STAT3- and C/EBP-dependent fashion. Int J Clin Pharmacol Ther. 2007;45:319–327. doi: 10.5414/cpp45319. [DOI] [PubMed] [Google Scholar]