Table 2.
Synthesis of tripeptides.a
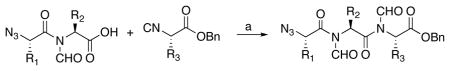 | ||||
|---|---|---|---|---|
| Entry | Dipeptide | Isonitrile | Product | Yield |
| 1 | 13 |
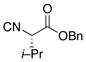 16 |
 19 |
65% |
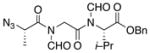
| 2 | 14 |
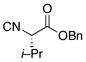 16 |
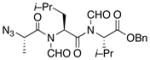 20 |
55% |
| 3 | 15 |
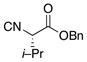 16 |
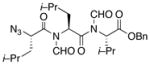 21 |
55% |
| 4 | 12 |
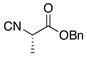 22 |
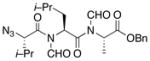 23 |
52% |
| 5 | 15 |
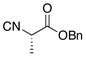 22 |
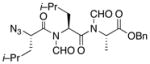 24 |
53% |
Key: (a) microwave, 120 °C, CHCl3, 2,6-dimethyl thiophenol.
