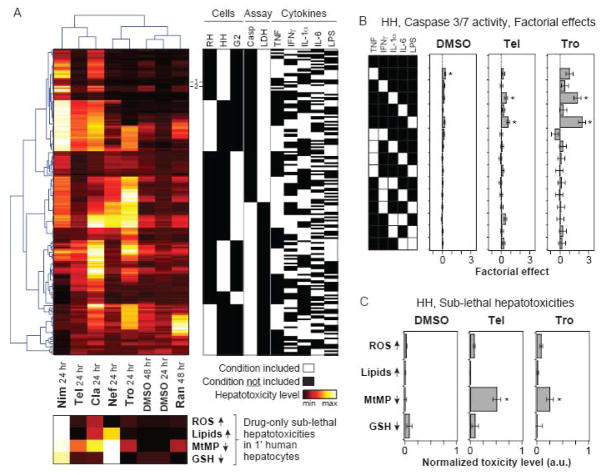
Figure 3.
Hierarchical clustering of the drug-cytokine mix hepatotoxicity compendium. (A) The drug-cytokine mix combinatorial hepatotoxicity compendium was fused across all cell systems and assay types into a single data matrix, which was then subjected to two-way Pearson clustering (top left; see Methods for additional details). First, clustering was used to re-sort a matrix of 192 “experimental” conditions, comprised of combinations of three cell systems, two assay types, and five cytokine treatment variables (top right). Second, this clustering was used re-sort to a sub-lethal hepatotoxicity data matrix of eight drug conditions and four drug (only)-induced sub-lethal hepatotoxicities (bottom). The sub-lethal hepatotoxicities (measured by quantitative imaging in primary human hepatocytes; see Figure S16) are plotted in the bottom heatmap using linear color-scales indexed separately to the minimum and maximum observed toxicity value for each assay type. (Note that the MtMP and GSH assay scales are inverted compared to Figures S16K-L.) Conditions used for the large-scale primary human hepatocyte toxicity study (see Figure 4) are noted: (1) no cytokines and (2) TNF, IL-1α, IL-6, and LPS. (B) Factorial effects ± errors of all one- and two-cytokine effects from the caspase 3/7 activity data at t = 24 hr in primary human hepatocytes for DMSO control, telithromycin, and trovafloxacin drug treatments. Statistically significant factorial effects (see Methods and Figure S12) are labeled (*). (C) Sub-lethal hepatotoxicities measured in primary human hepatocytes treated with DMSO control, telithromycin, or trovafloxacin are plotted on a normalized scale as in panel (A). Data are presented as mean ± SEM of five biological samples. For each assay type, treatments significantly different from the DMSO control are labeled as significant (*) if P < 0.05 by a Student’s t test. Abbreviations: RH, primary rat hepatocytes; HH, primary human hepatocytes; G2, HepG2 cells; Cla, clarithromycin; Tel, telithromycin; Nef, nefazodone; Tro, trovafloxacin; Nim, nimesulide; Ran, ranitidine; ROS, reactive oxygen species; MtMP, mitochondrial membrane potential; GSH, glutathione.