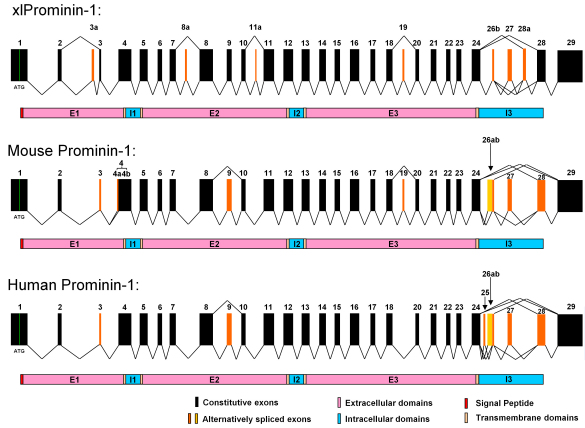
Figure 6.
Comparison of exon organization of prominin-1 gene from X. laevis, mouse, and human. It appears that the gene structure of prominin-1 is evolutionarily conserved in these animals. Alternative splicing of the prominin-1 gene is seen in all three animals, however, with considerable differences in the choices of alternative exons and the splicing patterns. Homologous exons of xlProminin-1 and mouse and human prominin-1 are aligned for comparison of their exon organization. Constitutive exons are marked in black. Alternatively spliced exons are marked in orange. Spliced forms identified in cDNA clones are indicated by jointed lines. Note that homologous exons are assigned with the same number to maintain consistency with preexisting nomenclature [2,31]. Exons 26b and 27 are conserved and alternatively spliced in all three species. Alternative exons 3a, 8a, 11a, and 28a of xlProminin-1 are not found in mouse or human prominin-1. Splicing of alternative exons 4a results from using an alternative 3′ splice site in exon 4, and is only observed in mouse prominin-1 [31]. Alternative exon 19 of xlProminin-1 is alternatively spliced in mouse prominin-1, but no evidence has been found that this exon is alternatively spliced in human prominin-1. Alternative exon 25 of human prominin-1 is not found in prominin-1 from the mouse or X. laevis. The alternative exon 26a of mouse prominin-1 and the alternative exons 25 and 26a of human prominin-1 are not found in xlProminin-1. Exons 3, 9, and 28 of mouse prominin-1 are alternative, but appear to be constitutive in X. laevis. The region encompassing exons 24 to 28 of xlProminin-1 and homologous sequences of the prominin-1 gene from the mouse and human are regions of extensive alternative splicing. Diagrams of translated proteins are aligned with their coding sequences. Divisions of proteins by predicted transmembrane domains are marked.