Abstract
The medial prefrontal cortex (mPFC) has been implicated in various attentional functions. This experiment examined the involvement of mPFC subregions in the allocation of attention in learning and action as a function of the predictive accuracy of cues. Rats with dorsal (encompassing anterior cingulate, prelimbic, and infralimbic cortices) or ventral (encompassing mainly infralimbic and dorsopeduncular cortices and tenia tecta) mPFC lesions were trained in a multiple-choice discrimination task in which operant nosepoke responses to some visual cues were consistently (100%) reinforced (CRF) with food, whereas responses to other visual cues were partially (50%) reinforced (PRF). In challenge tests designed to assess attention in the control of action, responding was directed more to CRF cues than to PRF cues in sham and dorsal mPFC-lesioned rats, but ventral mPFC-lesioned rats showed similar levels of responding to both CRF and PRF cues. Nevertheless, when given a choice between simultaneously presented CRF and PRF cues in a cue competition test, all groups responded more to CRF cues. In a subsequent Pavlovian overshadowing phase, designed to assess attention in the acquisition of new learning, previously-trained CRF cues overshadowed conditioning to novel auditory cues more than did PRF cues in dorsal mPFC-lesioned rats, whereas the opposite pattern was observed in sham and ventral mPFC-lesioned rats. These results suggest a dissociation within the mPFC in the use of reinforcement prediction information to allocate attention for new learning and for the control of action.
Keywords: attention, associative learning, medial prefrontal cortex, overshadowing, partial reinforcement
Considerable animal research demonstrates important roles for the medial prefrontal cortex (mPFC) in attention. Effects of manipulations of mPFC function have been demonstrated with a number of tasks, including 5-choice serial reaction time (5CSRT; e.g., Muir, Everitt, & Robbins, 1996), signal discrimination (e.g., McGaughy, Kaiser, & Sarter, 1996), and attentional set-shifting procedures (Birrell & Brown, 2000; McGaughy, Ross, & Eichenbaum, 2008). The results of such studies suggested that mPFC may modulate many aspects of attention.
Maddux et al. (2007) noted a distinction between the role of mPFC in attention in controlling action and in modulating new learning. Whereas rats with lesions of cholinergic input to the mPFC were impaired in responding under conditions of attentional challenge in a 5CSRT task, they showed normal surprise-induced enhancements of cue associability in a learning situation. A key feature of that study was the manipulation of cue validity. Many theorists (e.g., Le Pelley, 2004; Mackintosh, 1975; Pearce & Hall, 1980) have stressed the importance of the predictive validity of cues in determining the allocation of attention to those cues in the framework of associative learning. Interestingly, within some theories (e.g., Pearce & Hall, 1980), the effects of cue validity might differ depending on whether the cue is being used in new learning or in controlling previously-acquired actions. Casually speaking, whereas for purposes of selecting for action it is likely to be adaptive to direct one's attention to the most reliable predictors of future significant events, it may be counterproductive to focus attention on such events when one is acquiring new information about the environment. In a learning context, directing attention to events whose consequences are already known might waste resources that would be better allocated to cues whose consequences are less well understood. Thus, attention might modulate stimulus processing independently within systems devoted to changes in learning rate and those involved in action selection.
Maddux et al. (2007) first trained rats on a 5CSRT task, which has been extensively used to assess selective, sustained visual attention in rats (Bushnell, 1998; Robbins, 2002). On each trial, a rat could potentially earn food reward by poking its nose into whichever of five response ports was briefly-illuminated. Nosepokes to some of the ports were consistently reinforced (CRF), whereas nosepokes to other ports were reinforced on only half of the trials (partial reinforcement, PRF). In accordance with predictions of learning theories such as those of Mackintosh (1975), we expected that in intact rats, consistently reinforced (CRF) cues would command more attention in the performance of this task, relative to partially reinforced (PRF) cues. After acquisition of the baseline task, aspects of it, such as cue duration or the duration or timing of task ready signals, were altered so as to increase attentional demands, in ‘challenge’ test sessions. Sham-lesioned rats responded more accurately to CRF than to PRF cues under baseline conditions, and showed substantial disruptions in responding when challenged. However, rats with 192IgG-saporin lesions of mPFC failed to allocate responding to CRF and PRF cues differentially under baseline conditions, and in the challenge tests showed even larger impairments in responding than sham-lesioned rats.
Maddux et al. (2007) then evaluated the ability of the CRF and PRF port cues to control new learning in the same rats. Each rat received Pavlovian pairings of two separate compound cues with the delivery of a new reward that was larger and more palatable than the one used in the prior training. One compound comprised a port cue that had been previously trained with CRF contingencies and a novel tone, and the other comprised a former PRF port cue and a different novel tone. According to Pearce and Hall's (1980) model of associative learning, cues that consistently predict future events gradually lose their associability, that is, their ability to enter into new learned associations, whereas the associability of unreliable predictors is maintained. Thus, within this model, for purposes of new learning, more attention would be allocated to PRF cues than to CRF cues. In the context of Maddux et al.'s (2007) experiment, the PRF cue would be expected to overshadow conditioning to its tone partner more than the CRF cue. Sham-lesioned rats indeed showed this pattern of data in testing. Thus, for those rats, PRF cues were more effective than CRF cues in modulating new learning, but, as observed in the previous 5CSRT tests, CRF cues were more effective than PRF cues in controlling previously well-learned responses. Notably, although rats with mPFC lesions had shown abnormal allocation of responding to CRF and PRF cues and substantial performance impairments in challenge tests in the 5CSRT task, their performance in the new learning task was similar to that of sham-lesioned rats. Interestingly, rats with lesions of another cortical area, the posterior parietal cortex (PPC), showed the opposite pattern of impairments; performance of those rats in the 5CSRT tests was unaffected, but they failed to show differential overshadowing in the new learning task. Thus Maddux et al. (2007) proposed that mPFC was critically involved in attention for action but not in attention for new learning, whereas the opposite was true for PPC.
In the experiment reported here, we examined the effects of more extensive excitotoxic (NMDA) lesions of mPFC, which were not specific to its cholinergic innervation, on both ‘action’ and ‘learning’ tasks similar to those used by Maddux et al. (2007). However, unlike in that study, our multiple-choice discrimination performance task did not place a premium on rapid reaction times, and we assessed the allocation of attention to CRF and PRF port cues both by examining the effects of reductions in port cue durations and by examining performance in a combined cues test, in which rats were faced with a choice between CRF and PRF cues presented simultaneously. Finally, we assessed the ability of CRF and PRF cues to modulate new learning by using procedures identical to those of Maddux et al. (2007).
A critical feature of this experiment was the comparison of the effects of two mPFC lesions, a dorsal lesion, which damaged much of anterior cingulate (ACC), prelimbic (PL), and infralimbic (IL) cortex, and a ventral lesion, which damaged the tenia tecta (TT), dorsopeduncular cortex (DP), and IL, as well as parts of PL. Consistent with recent views of functional anatomical specialization within the mPFC (e.g., Heidbreder & Groenewegen, 2003), a number of investigators have found evidence for specialization of function within subregions of mPFC along a dorsal-ventral axis (e.g., Passetti, Chudasama, & Robbins, 2002; Chudasama, Passetti, Rhodes, Lopian, Desai, & Robbins, 2003; Murphy, Dalley, & Robbins, 2005). For example, using the 5CSRT task, Passetti et al. (2002), Chudasama et al. (2003), and Murphy et al. (2005) found different effects of lesions or inactivation procedures that targeted anterior cingulate cortex and those that targeted prelimbic (PL), infralimbic (IL), or combined PL+IL cortical areas. More recently, Maddux and Holland (2010) found multiple dissociations among the effects of ventral mPFC lesions that targeted DP and TT, and dorsal mPFC lesions that targeted IL and PL, on aspects of performance in the 5CSRT task. For example, rats with ventral lesions tended to ‘guess’ more (increased errors) whereas rats with dorsal lesions tended to ‘give up’ (more trials with no responses) when challenged. Most notably, although ventral lesions disrupted the way rats allocated behavior to CRF and PRF cues, as Maddux et al. (2007) noted with cholinergic lesions of mPFC, rats with dorsal mPFC lesions showed no such abnormality. Given the role of cue validity in the influence of attention in both action and new learning, comparison of the effects of the two lesions on both behavioral functions is especially important.
Materials and Methods
Subjects
Thirty-two male Long-Evans rats (Charles River Laboratories, Raleigh, NC), weighing between 300-325 grams when they arrived in the laboratory vivarium, were used in this experiment. The rats were housed individually, and were maintained at 85% of their ad libitum weights by restricting their access to food. The laboratory viviarium was illuminated from 7 A.M. to 7 P.M.
Surgical procedures
Surgery was performed prior to behavioral training. Bilateral neurotoxic lesions of the mPFC were made under aseptic conditions, using isoflurane anesthesia. Lesions were made using NMDA at a concentration of 17.5 mg/ml in Dulbecco's PBS (Sigma, St. Louis, MO). Dorsal lesions were made using the following coordinates: AP: +3.0, ML: +/− 0.7, and DV: −4.5 and −3.5 from the skull surface. Ventral lesions were made using the following coordinates: AP: +3.0, ML: +/− 0.6, and DV: −5.0 and −4.0 from the dura. For the dorsal-lesioned rats, a volume of 0.10 μl was infused at the −4.5 DV site over a 60-s period and a volume of 0.20 μl was infused at the −3.5 DV site over a 120-s period, using a 2.0 μl Hamilton syringe. For the ventral-lesioned rats, a volume of 0.15 μl was infused, using a 2.0 μl Hamilton syringe, at each injection site over a period of 90 s. The needle was left in place at each injection site for 1 min before infusing and 4 min after infusing the toxin. Sham-lesioned rats had drill holes drilled through the skull at the injection site but no needle lowered into the brain. All rats were given a 14 day recovery period after surgery before behavioral training began.
Apparatus
The behavioral training apparatus consisted of four individual 5CSRT chambers (25 cm × 25 cm × 25 cm; Cambridge Cognition, Cambridge, UK). Each chamber had aluminum front and side walls and a clear acrylic back wall and top. Nine 2.5 cm × 2.5 cm × 4 cm (deep) stimulus-response ports were spaced 2.5 cm apart, and centered on the front, curved wall of the chamber, 2 cm above a grid floor. Of these 9 ports, the center port and the two rightmost and leftmost ports were each covered with opaque metal plates. Thus, we used only two ports left-of-center and two ports right-of-center. A 3 W lamp at the back of each port provided illumination as the port cues; responding in the ports was detected with infrared phototransistors. A recessed food cup was located in the center of the back wall of the chamber. This food cup was fitted with a lamp that was illuminated when food pellets were delivered and a transparent acrylic flap to detect food cup entries. A speaker was mounted on the top of each chamber. Each chamber was enclosed in a sound-attenuating box where ventilation fans provided masking noise (70 dB). An overhead bank of infrared LEDS provided background illumination for video monitoring and recording, but this illumination was invisible to the rats. A television camera was mounted within each box to allow for video recording during behavioral training and testing.
Behavioral training procedures
The rats were first familiarized with the apparatus in four sessions. In the first 15-min session, ten 45-mg grain food pellets (Test Diet, Richmond, IN) were present in the illuminated food cup, and the acrylic flap to the food cup was propped open. In the next 15-min session, five food pellets were again placed in the illuminated food cup, but the flap door was not propped open. In addition, all four port lights were continuously illuminated, and two food pellets were placed in each response port. In each of the next two 32-min sessions, there were 16 deliveries of a food pellet, accompanied by a 1-s illumination of the food cup light, to train the rats to collect food from the food cup.
Thereafter, the operant training of the nosepoke response to illuminated stimulus-response ports began. The final duration of the port light cue illumination during baseline training sessions was 5 s. Each of the four ports was equally likely to be illuminated on any trial. The first response to the correct port during port illumination was reinforced with the delivery of a food pellet to the food cup (accompanied by a 1-s illumination of the food cup) and the darkening of the port. If no correct response was made before the end of the 5-s response window, the port light turned off and the trial ended. Responses to the ports that were not illuminated on a trial were recorded as errors but had no scheduled consequences. Sixty trials were presented in random order at predetermined intervals within each 32-min session; trial delivery was not affected by the rats' behavior. Rats were shaped to this procedure gradually but all rats received the same treatment (the shaping was not individualized). Between sessions, the duration of port illumination was reduced, and the number of trials was increased, from 30 s (8 of each port cue per session) in the first four training sessions to 5 s (15 of each port cue per session) over the course of 12 sessions.
After the 5-s cue duration level was initially reached, the rats required 6 training sessions at the 5-s level to perform this task at a stable baseline criterion of approximately 80% accuracy. After this stable baseline performance was reached, the final reinforcement contingencies were introduced. For each rat, two of the ports were designated for CRF (100% reinforcement) and two for PRF (50% reinforcement), spatially counterbalanced across rats. In all cases, both CRF ports were on one side of the center port and both PRF ports were on the other side. Each 32-min session included 56 total trials, with 14 trials of each port cue. CRF trials were identical to those presented previously. Responding on half of the trials with the PRF port cues were reinforced as before, but on the other half of those trials, correct responses terminated the illumination of the port light but did not produce food delivery nor food cup illumination.
Cue competition test
After 8 CRF/PRF training sessions, all rats received a cue competition test session, to determine the effects of reinforcement contingency on the allocation of attention. This 32-min test session included 56 total trials and was conducted in extinction. In it, 28 compound cue trials (consisting of one CRF cue and one PRF cue illuminated simultaneously) and 28 element cues (14 trials of the CRF cue alone and 14 trials of the PRF cue alone) were presented. Element and compound cues were all illuminated for 5 s, and remained illuminated for the full 5 s, even if the rat made a nosepoke response to the illuminated port. Following this test session, all rats received one baseline CRF/PRF retraining session before being tested in the cue duration challenge test.
Cue duration challenge test
This test was designed to examine the effect of shortened port light duration on the probability of responding. It was identical to baseline CRF/PRF training sessions, except the port lights were only illuminated for 1 s. Responses that occurred within the usual 5-s response window initiated by port light onset were counted.
Overshadowing test
This test sequence was designed to assess the effects of lesion and cues' prior reinforcement contingency on the acquisition of new learning. After two retraining sessions of baseline CRF/PRF multiple-choice discrimination training to re-familiarize the rats with the previously trained reinforcement contingencies, all rats received four Pavlovian conditioning sessions in which two auditory-visual compound stimuli were consistently reinforced with the delivery of a new, larger, and more palatable reinforcer. On CRF trials, a compound of one of the previously established CRF port cues and either an 80-dB 1500 Hz (low) tone or an 80-dB 4000 Hz (high) tone (counterbalanced) was presented for 5 s, and followed immediately by delivery of six 45-mg fruit punch pellets (Test Diet, Richmond, IN) and illumination of the food cup light as the pellets were delivered. PRF trials were similar, except that the 5-s compound CS was composed of one of the previously trained PRF port lights and the other tone. It is important to recognize that the CRF and PRF designations refer to the past reinforcement history of the port cues, and not the Pavlovian contingencies present in this phase, which were the same for both compounds.
Following the four Pavlovian conditioning sessions, all rats received two 32-min test sessions, each with 8 total trials. The first test assessed conditioning to the tones and the second test assessed conditioning to the port lights. The first test included four presentations of each 5-s tone, randomly intermixed, and the second test included four presentations of each of the two 5-s port lights, randomly intermixed. Both test sessions were conducted in extinction.
Response measures
For baseline discrimination training and cue duration challenge sessions, the primary measures of performance were the percentage of trials on which at least one correct response was made, the percentage of trials on which at least one error was made, and the percentage of trials on which no responses occurred (omissions). In each case, the time window in which these responses were defined was the 5-s interval that began with the illumination of a port light. Because on each trial there were 3 incorrect ports and one correct port, the chance percentages of responses made were 25% for correct responses and 75% for errors. In the cue competition test and the final port light test, the primary measure was the rate of the nosepoke response (nosepokes/min) during the entire 5-s interval of port light illumination. Especially when relatively small numbers of trials are involved, response rate is typically more sensitive to variations in conditioning than response probability. (The use of rate measures was not appropriate in the discrimination acquisition and cue duration test sessions because in those sessions the first correct response led to reinforcement and termination of the trial, whereas any number or errors could be made without consequence). The primary response measure for the final tone test was the percentage of time the rat spent with its head in the food cup during the 5-s CS minus the percentage of time during the 5-s period immediately preceding the CS. Finally, we also reported pre-CS responding (response probabilities or rates in the 5-s empty interval prior to stimulus onset) for all test sessions. Note that trials were not signaled in any way nor dependent on prior responding or event delivery. Separate ANOVAs were conducted for each response measure. Post-hoc comparisons used Tukey's Honestly Significant Difference (HSD) test, with a Spjotvoll-Stoline correction for unequal ns. The level of statistical significance adopted was p < .05; p values for nonsignificant differences that approached significance were also reported.
Histological procedures
After completion of behavioral testing, the rats were deeply anesthesized with isoflurane, and perfused transcardially with 0.9% saline followed by 10% (v/v) formalin. The brains were removed and stored in 0.1 M PBS with 20% (w/v) sucrose, 2.5% (w/v) formaldehyde, and 1.25% (w/v) dimethyl sulfoxide (DMSO) for 48-72 hours. Sections (40 μm) from each brain were collected on a freezing microtome. Every third section was mounted on glass microscope slides and Nissl-stained to verify lesion placement.
Results
Histology
Lesioned rats had to sustain bilateral damage from at least +3.20 to +2.20 mm anterior to bregma, according to the atlas of Paxinos & Watson (1997), to qualify as acceptable for inclusion in the behavioral analysis. One rat was excluded because its lesion was too far posterior, one because its lesion went far beyond the intended mPFC regions, and four rats were excluded from the dorsal lesion group because their lesions included either unilateral or bilateral damage to the ventral DP and TT regions. The presence or absence of any damage to TT completely distinguished ventral from dorsal lesions; two independent observers, one of whom was blind to intended lesion type, inspected the lesions and agreed on all judgments regarding the presence or absence of TT damage. Little or no damage was observed in any of the sham-lesioned rats. In all, we accepted 7 dorsal-lesioned, 11 ventral-lesioned, and 8 sham- lesioned rats for behavioral analysis.
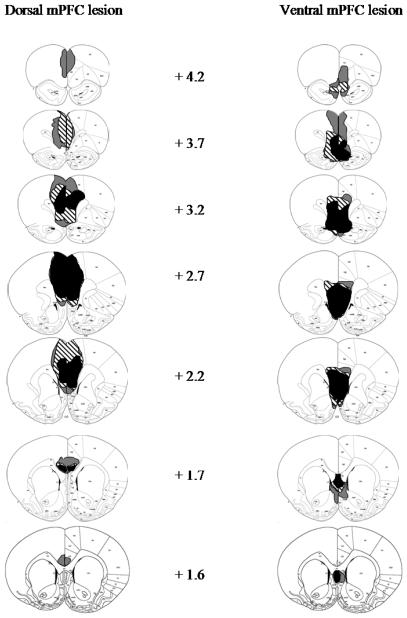
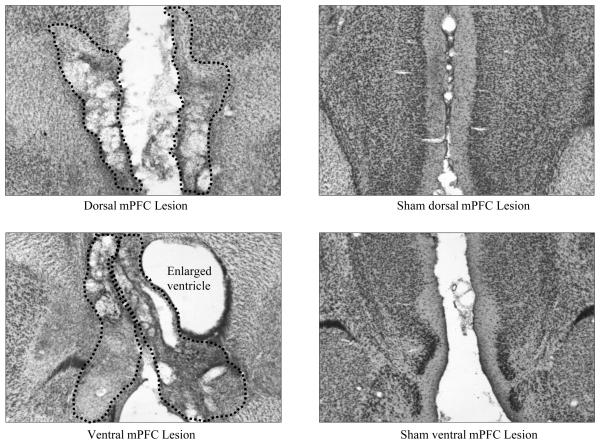
Figure 1 shows the largest, smallest, and representative dorsal and ventral mPFC lesions, and Figure 2 shows photomicrographs of dorsal, ventral, and sham mPFC lesions. Although the accepted dorsal and ventral lesions were clearly differentiable, there was substantial overlap in their extent as well. All rats sustained comparable and nearly complete damage to IL, and both lesion groups showed substantial damage to PL, albeit somewhat more in the dorsal group. By contrast, damage to ACC (and to a lesser extent, secondary motor cortex, M2) was substantial in most anterior-posterior planes in the dorsal group, but minimal in most rats in the ventral group, and damage to DP, TT, and portions of the medial septal area was found almost exclusively in the ventral group. In one ventral lesion, more extensive dorsal damage was observed along the injector path.
Figure 1.
Extents of minimum (black), maximum (gray), and representative (stripes) dorsal (left) and ventral (right) mPFC lesions at various distances anterior to bregma. Coronal sections from Paxinos and Watson (1997).
Figure 2.
Photomicrographs of dorsal (top) and ventral (bottom) neurotoxic (left panels A and C) and sham (right panels B and D) mPFC lesions. The dotted lines signify the boundaries of the lesion. The hole directly to the right of the ventral lesion in panel C is an enlarged ventricle.
Acquisition of CRF multiple-choice discrimination task
All rats gradually learned to make nosepoke responses to the illuminated port lights, responding correctly on about 70% of the trials by the last sessions of this phase. A mixed ANOVA of percentage trials with a correct response with lesion as the between-group variable and session as the repeated within-subject variable was performed on the data from the 6 sessions in which the final baseline target duration of the port light stimulus (5 s) had been reached. This ANOVA revealed only a significant main effect of session, F(5,115) = 20.50, p < .001, reflecting continued acquisition of the task. Although the overall performance of both dorsal- (59.5 ± 9.9 %) and ventral- (62.8 ± 5.1%) lesioned rats was numerically inferior to that of the sham-lesioned rats (76.5 ± 2.5%), neither the lesion effect nor lesion X session interaction was significant, ps > .10.
Introduction of PRF cues to multiple-choice discrimination task
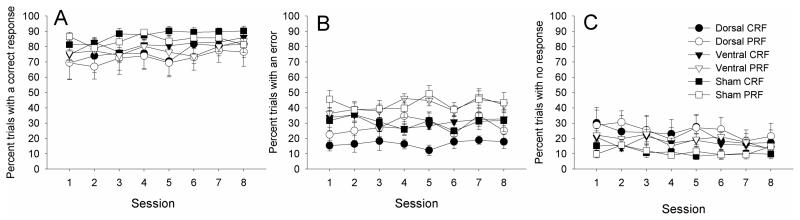
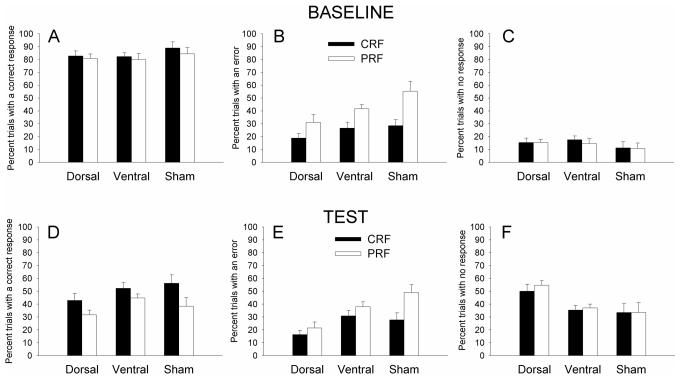
After the six training sessions in which only CRF cues were presented, partial reinforcement contingencies were added for eight CRF/PRF training sessions. Separate ANOVAs were conducted for correct responses, errors, and omissions (Figures 3A, 3B, and 3C, respectively), each with the between-subject factor of lesion and the within-subject repeated factors of contingency (CRF vs. PRF) and session (8 sessions).
Figure 3.
Correct (left panel A), error (middle panel B), and omission (right panel C) nosepoke responding in the CRF/PRF multiple-choice discrimination training sessions. The port light duration was the baseline level of 5 s. CRF = consistently reinforced; PRF = partially reinforced.
Rats made more correct responses, F(1,23) = 12.21, p =.002, and fewer errors, F(1, 23) = 74.36, p <.001, to the CRF than to the PRF port lights, and omitted responding entirely (marginally) more on PRF than CRF trials, F(1, 23) = 4.18, p = .053. In the ANOVAs of correct responses and omissions, there were main effects of session, Fs(7,161) > 3.90, ps < .001. This main effect of session was not significant for the analysis of errors, but the interaction of reinforcement contingency with session was significant for error responses, F(7,161) = 3.60, p = .001, and marginally significant for correct responses, F(7,161) = 2.06, p = .051. In the analyses of correct responses and of omissions, neither the main effects of lesion nor any of its interactions were significant. However, in the analysis of errors, there was a main effect of lesion, F(2,23) = 4.68, p = .020. A post-hoc Tukey's HSD test revealed that the dorsal-lesioned group of rats showed fewer errors than both the ventral- (p = .049) and the sham- (p = .044) lesioned groups of rats, which did not differ from each other (p = .998).
Cue competition test
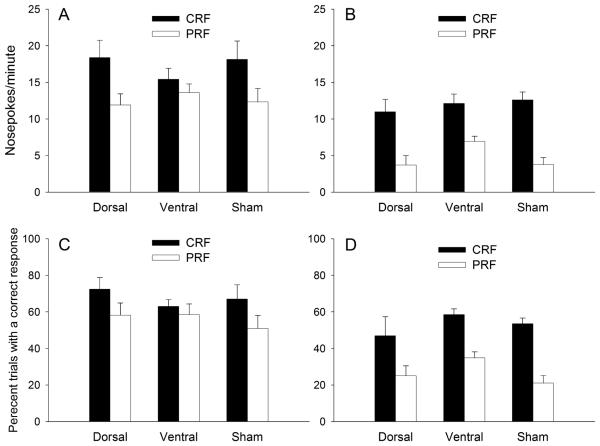
The first assessment of attention in the control of action in this experiment was the cue competition test, which included both compound choice trials that pitted a CRF cue and a PRF cue against each other in a simultaneous compound, and CRF- and PRF-cue alone element trials. This test was conducted under extinction conditions; no food was delivered and all ports remained illuminated for the full 5-s duration, regardless of whether or not the rat made a nosepoke response. Thus, responses could be allocated to both choice cues on compound trials,. Nosepoke rates for both the element and compound trials are shown in Figures 4A and 4B, respectively.
Figure 4.
Panels A and B show port light nosepoke rates for the element and compound trials (respectively) in the cue competition test. Panels C and D show the percentage correct responses for the element and compound trials (respectively). The port light duration was 5 s. CRF = consistently reinforced; PRF = partially reinforced.
Consistent with Maddux and Holland's (2010) observations, on element trials, both sham and dorsal-lesioned rats responded more to CRF than to PRF port lights, but ventral-lesioned rats did not show differential rates of nosepoking to the CRF vs. PRF ports (Figure 4A). These observations were confirmed by planned comparisons on each separate lesion group, contrasting CRF vs. PRF responding: sham: F(1,23) = 6.51, p = .018; dorsal: F(1,23) = 7.12, p = .014; ventral: F(1,23) = .91, p = .351. This apparent insensitivity to reinforcement contingency by the ventral-lesioned rats disappeared however, when they were presented with the CRF and PRF port lights simultaneously on compound trials. On such compound trials, all groups of rats displayed comparable discrimination between the CRF and the PRF ports (Figure 4B). These observations were confirmed by planned comparisons on each separate lesion group, contrasting CRF vs. PRF responding: sham: F(1,23) = 22.73, p < .001; dorsal: F(1,23) = 13.40, p = .001; ventral: F(1,23) = 10.81, p = .003. Thus, ventral-lesioned rats were not completely insensitive to reinforcement contingency. Instead, it appears that under some circumstances (for example, when only one port light is presented at a time), they fail to use reinforcement contingency information to determine responding.
Errors were considerable less frequent than correct responses in this test (Table 1). A lesion X error type (same vs. opposite side) X trials type (CRF vs. PRF) ANOVA showed that on element trials, the most frequent errors were to the port adjacent to the illuminated one, F(1, 23)=58.73, p<.001. In addition. rats were more likely to respond in error to a port on the other side on PRF trials than on CRF trials (error type X trial type interaction, F(1, 23)=23.15, p<.001. That is, although rats were most likely to err on the same side as the illuminated port, that tendency was modulated by a tendency to make CRF errors more than PRF errors. Unlike correct responses, the pattern of errors in this test was unaffected by lesion; no effects or interactions with lesion were significant, Fs<1, ps>.453. Probably because there were two illuminated ports available on combined cue tests, responses to unilluminated ports were negligible on compound trials (< 3 responses/min) and did not differ across conditions, ps >.610
Table 1.
Error responses to element cues in the combined cue test
| CRF trials | PRF trials | |||
|---|---|---|---|---|
| Same | Other | Same | Other | |
| Dorsal | 5.39±1.29 | 0.19±0.13 | 2.47±0.82 | 0.69±0.29 |
| Ventral | 5.62±0.96 | 0.75±0.19 | 1.24±0.47 | 2.42±0.81 |
| Sham | 5.46±1.13 | 0.33±0.16 | 4.08±1.37 | 1.71±0.65 |
Notes. Entries are mean±SEM responses/min. CRF and PRF trials refer to presentations of the cues that were trained with consistent or partial reinforcement, respectively. Same errors refer to responses to the unilluminated port on the same side of the chamber centerline as the illuminated (correct) port, and Other errors refer to responses to the corresponding port on the other side. Thus, a Same error on a CRF trial was a responses to the unilluminated CRF port, whereas an Other error on that trial was a response to an unilluminated PRF port.
Pre-CS responding in the combined cue test was very infrequent, but ANOVA showed a significant main effect of lesion, F(2,23) = 4.47, p = .023. Post-hoc contrasts showed that pre-CS responding of the dorsal-lesioned rats (0.22 ± 0.18 responses/min) was significantly (p=.038) lower than that of the ventral-lesioned (1.30 ± 0.38 responses/min) rats, but not significantly (p=.107) lower than responding in the sham-lesioned (1.47 ± 0.99 response/min) rats.
Although we chose rate of responding as the most sensitive measure in this test, we also analyzed the percentage of trials on which correct responses occurred (Figures 4C and 4D), the same measure used in acquisition and the cue duration test. This measure showed patterns similar to those observed with the response rate measure. On element trials, the dorsal- and sham-lesioned rats responded correctly more on CRF trials than on PRF trials, p=.019, whereas rats in the ventral group did not, p=.524. By contrast, on compound trials, all three groups showed more correct responding on CRF than PRF trials, ps< .013.
Cue duration challenge
In this test session, the duration of the port cues was reduced from 5 s to 1 s. This manipulation degraded performance compared to baseline (5 s) performance in all groups, but the dorsal-lesioned rats showed additional impairments, omitting responding entirely on more trials than either ventral- or sham-lesioned rats, which did not differ in this measure. Separate mixed ANOVAs with the between-subject factor of lesion and the repeated within-subject factors of test (baseline vs. test) and contingency (CRF vs. PRF) were performed on the percentages of trials with correct (Figures 5A, 5D), error (Figures 5B, 5E), and no (omission; Figures 5C, 5F) responses. Overall, rats made correct responses on fewer trials, F(1,23) = 240.71, p < .001, and omitted responding entirely on more trials, F(1,23) = 105.48, p < .001, in the test than in the baseline session. Thus, the cue duration reduction was an effective challenge to performance. There was a main effect of reinforcement contingency on both correct responses and errors; rats made more correct responses, F(1,23) = 16.44, p < .001, and fewer errors, F(1,23) = 64.24, p < .001, to the CRF than to the PRF ports. For correct responding, reinforcement contingency interacted with test; the difference in correct responses to CRF vs. PRF ports was greater in the test than in the baseline session, F(1,23) = 6.49, p = .018, suggesting that the reduced port light duration placed an added emphasis on the distribution of correct responding based on reinforcement contingency. There were no lesion effects or lesion interactions on correct responding. However, there were significant lesion effects for both errors and omissions.
Figure 5.
Correct (left panels A and D), error (middle panels B and E), and omission (right panels C and F) nosepoke responding in the baseline (top panels) and test (bottom panels) sessions of the cue duration challenge. The duration of the port light stimulus was reduced from the baseline level of 5 s to the test level of 1 s. CRF = consistently reinforced; PRF = partially reinforced.
First, there was a main effect of lesion on the number of errors made, F(2,23) = 5.83, p = .009. Post-hoc Tukey's HSD tests revealed that the dorsal-lesioned rats made significantly fewer errors than the sham-lesioned rats (p = .010) and numerically but not significantly fewer errors than the ventral-lesioned rats (p = .093), but that sham and ventral-lesioned rats did not differ from each other (p = .51). Note however that this effect was not specific to the challenge itself: the dorsal lesioned rats also showed fewer errors in training and in the baseline session of this challenge test (the lesion X test interaction was not significant). Although at first glance it seems surprising that lesioned rats would show fewer errors than sham-lesioned rats, this observation is readily interpretable when taken together with the omission data (below); in the absence of a correct response, the dorsal-lesioned rats omitted responding entirely rather than commit errors. The lesion X reinforcement contingency interaction was also significant; the effects of the lesions on CRF and PRF errors differed, F(2,23) = 6.63, p = .005. Tukey's HSD tests showed that on CRF trials, dorsal-lesioned rats made significantly fewer errors than ventral-lesioned rats (p = .039) and marginally fewer errors than sham-lesioned rats (p=.056), which did not differ (p = .99), whereas on PRF trials, dorsal-lesioned rats made fewer errors than ventral-lesioned rats (p =.008), which in turn made fewer errors than sham-lesioned rats (p = .009).
Second, there was a lesion X test interaction on the number of omissions made: the difference in the number of omissions from baseline to test varied across the lesion groups, F(2,23) = 3.90, p = .035. Post-hoc Tukey's HSD tests showed that the dorsal-lesioned rats were particularly impaired by the challenge test: the difference in omissions from baseline to test was similar for sham and ventral-lesioned rats, but greater for dorsal-lesioned rats. This observation was supported by pairwise comparisons, in which all groups displayed similar levels of omissions in the baseline sessions (ps > .870), but in test, dorsal-lesioned rats made more omissions than either ventral- (p = .034) or sham-lesioned rats (p = .010), which did not differ (p = .99).
Unlike in the combined cues test, a lesion X error type X trial type ANOVA of the distribution of errors across the different unilluminated ports showed no significant effects or interactions, ps > .222. Finally, as in the combined cue test, pre-CS responding differed across groups, F(2,23)=4.29, p=.026. Post-hoc Tukey's HSD comparisons showed that dorsal-lesioned rats (10.0±2.5%) had significantly (p=.049) fewer responses prior to stimulus onset than sham-lesioned rats (23.6±4.8%), and marginally (p=.069) fewer than ventrally-lesioned rats (22.7±2.7%).
Acquisition of Pavlovian conditioned food cup response
Four rats (1 dorsal-, 2 ventral-, and 1 sham-lesioned) failed to condition to either of the port light + tone compound stimuli, as measured by the percent time spent in the food cup during stimuli presentation, and were thus excluded from subsequent analysis. These rats showed no change in responding over the course of acquisition and overall elevation scores of zero or less. This left a total of 6 dorsal-, 9 ventral-, and 7 sham-lesioned rats in the final analyses. Over the four days of Pavlovian conditioning, these rats learned to approach and enter the food cup in response to the presentation of the port light + tone compound stimuli. Mixed ANOVAs with the between-subject factors of lesion and a counterbalancing variable, and the repeated within-subject factors of tone frequency and session were conducted for both percentage time in the food cup during the compounds and for elevation scores (percent time in food cup during CS presentation minus percent time in food cup during pre-CS period). The counterbalancing variable referred to the combination of tone frequency (high or low) with port light contingency (CRF or PRF) in creating the two compound stimuli used in Pavlovian training. These ANOVAs revealed only significant main effects of session, Fs(3,48) > 22.65, ps < .001. A comparable ANOVA of pre-CS time in food cup showed no reliable effects or interactions, ps> .249. Overall, the mean (±sem) percentage times in the food cup were 8.9±3.0, 5.2±1.3, and 7.1±1.5%, in dorsal-, ventral-, and sham-lesioned rats, respectively.
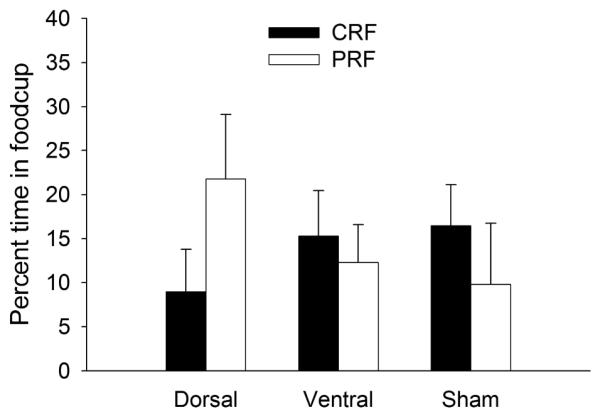
Tone overshadowing test
The tone test examined conditioning to the tones as an indirect measure of attention to the port lights. The more the port light captured attention for learning, the less the tone presented with it should be conditioned. Based on previous data (Maddux et al., 2007), we anticipated that in sham-lesioned rats the PRF cue would garner more attention than the CRF cue and thus learning about the tone partnered with the PRF cue during compound training would show less responding in this test than the tone partnered with the CRF cue. The question of interest was whether that pattern would be altered by the mPFC lesions. As portrayed in Figure 6, this pattern was apparently observed in sham and ventral-lesioned rats, but was significantly reversed in dorsal-lesioned rats.
Figure 6.
Food cup conditioning to the tones in the tone overshadowing test. The CRF/PRF designation in the legend refers to the previous operant reinforcement contingency of the port lights with which the tones were paired in Pavlovian training. CRF = consistently reinforced; PRF = partially reinforced.
A mixed ANOVA with the between-subject variables of lesion and the counterbalancing variable described previously, and the repeated within-subject variable of previous reinforcement contingency of the two port lights (CRF vs. PRF) that accompanied the tones in training, was conducted on the food cup conditioning elevation scores. This analysis revealed a significant interaction of lesion with reinforcement contingency, F(2,16) = 4.32, p = .032, indicating that the relative amounts of overshadowing produced by CRF and PRF cues were affected by the lesions. In the dorsal-lesioned rats, conditioning to the tone that had been trained in compound with the PRF port light was significantly greater than conditioning to the tone that had been trained with the CRF port light, p = .016. Thus, in those rats, the PRF cue overshadowed tone learning less than the CRF cue, contrary to expectations from the Pearce-Hall (1980) model. Moreover, this PRF-CRF difference was significantly greater in the dorsal-lesioned rats than in either the sham- (p = .016) or ventral- (p = .024) lesioned rats. Thus, the dorsal-lesioned rats clearly showed less overshadowing by the PRF port cue than the sham- or ventral-lesioned rats. However, although responding to the tone that had been trained in compound with the PRF port light was numerically lower than responding to the tone that had been trained with the CRF port light in those two groups, pairwise comparisons within each of them were not significant, ps > .10. This failure to observe significantly more overshadowing by PRF than by CRF cues in sham-lesioned rats, which we have observed in previous studies (Maddux et al., 2007), was unexpected, and was at least in part due to an apparent floor effect with one of the auditory cues. Indeed, the ANOVA revealed a significant interaction of the contingency effect with the counterbalancing variable, F(1, 16) = 5.84, p =.028. Subsequent contrasts of the PRF-CRF difference in the sham- and ventral-lesioned rats were significant in one counterbalancing condition, p =.006, but not the other, p =.286. However, the critical observation in the present test was the significant reversal of this pattern in the dorsal-lesioned rats.
The rats spent little time in the food cup prior to tone presentations (1.2±.7% to 6.3±2.6%), and there was no effect of lesion on that responding, F(2,19)= 1.686, p=.212.
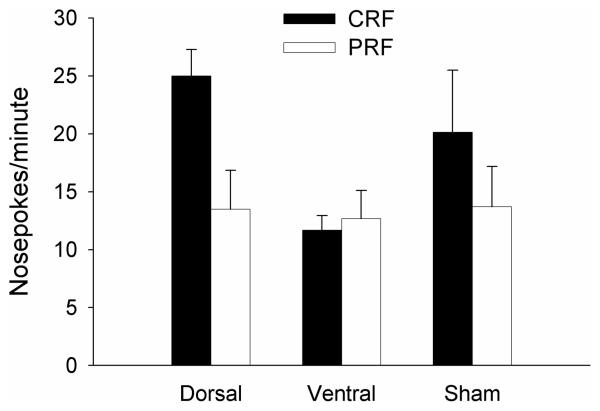
Port light test
The port light test was designed to reassess control of the nosepoke response to the CRF and PRF port lights (Fig. 7). Essentially, the rats maintained the pattern of responding observed to those ports prior to the Pavlovian training, in which ventral-lesioned rats failed to respond differentially to CRF and PRF ports whereas the other groups responded more to CRF than to PRF ports (e.g. Figure 4A). A mixed ANOVA with the between-subject variable of lesion and the repeated within-subject variable of previous reinforcement contingency revealed a significant main effect of reinforcement contingency, F(1,19) = 7.43, p = .013, and a marginal interaction of lesion with reinforcement contingency, F(2,19) = 3.22, p = .063. Planned comparisons of CRF to PRF responding showed reliably greater responding to the CRF port in the dorsal- and sham-lesioned groups, F(1, 19) = .003, but not the ventral-lesioned group, F(1, 19) = 0.10, p = .758. These data indicate that substantial operant nosepoke associations persisted throughout the intervening Pavlovian training.
Figure 7.
Port light nosepoke rates in the port light test that occurred after Pavlovian training and the tone overshadowing test. The CRF/PRF designation in the legend refers to the previous operant reinforcement contingency of the port lights. CRF = consistently reinforced; PRF = partially reinforced.
Errors (responses to an unilluminated port) were infrequent, but as in the combined cues test, were more likely to occur to the adjacent port (4.6±0.6 responses/min) than to ports on the other side (1.9±0.4) of the center line. ANOVA showed a significant effect of side, F(1, 19) = 11.46, p=.003, but no effect of lesion or its interactions, ps > .369. Pre-CS responding was less than 4 responses/min and did not differ significantly across groups, F<1.
Discussion
Throughout this study, rats with lesions to the mPFC that included the ventral-most regions, DP and TT, as well as IL/PL, failed to respond differentially to CRF and PRF cues when those cues were presented individually, including in the combined cues test, the cue duration challenge test, and in the final post-overshadowing test of port cue responding. By contrast, both sham-lesioned rats and rats with more dorsal mPFC damage, which left DP and TT intact but included substantial damage to ACC as well as to IL/PL, showed greater responding to CRF than PRF cues. Although rats with dorsal lesions of mPFC showed normal distribution of responding to CRF and PRF cues, they nevertheless showed performance impairments: they were more likely to omit responding entirely on a trial than either ventral- or sham-lesioned rats, which did not differ in that respect. These results, concerning the control of action by the port cues, are broadly consistent with Maddux and Holland's (2010) observations from rats with more limited (and more distinct) ventral and dorsal lesions of mPFC, and thus extend their results to somewhat different lesions, a discrimination procedure that does not put a premium on rapid responding during acquisition, and a combined cues choice test procedure.
Although, as in Maddux and Holland's (2010) report, rats with ventral mPFC lesions showed abnormal allocation of responding to CRF and PRF cues presented individually, other evidence from the present experiment indicated ventral-lesioned rats were normal in their sensitivity to those cues' different predictive relations with reinforcement. First, when CRF and PRF cues were presented simultaneously in the combined cues test, their allocation of nosepoke responses was similar to that of sham-lesioned rats. This observation is striking in its discrepancy with previous suggestions (in the context of human movement control) that ventral prefrontal regions are especially engaged when cue-guided response choice is required compared to when a cue simply drives visual action (e.g., Passingham & Toni, 2001). Second, PRF port cues appeared to overshadow conditioning to tones more than CRF port cues in both ventral- and sham-lesioned rats, although this difference was not significant in either group. This observation is consistent with previous data (Maddux et al., 2007) and the Pearce-Hall (1980) model, in which cues that are inconsistent in their prediction of reinforcement maintain higher levels of associability than consistent predictors, and hence may be more able to overshadow conditioning to other cues. Thus, ventral-lesioned rats appeared normal in their ability to alter cues' associability as a function of their predictive power, even when those cues failed to control differential responding in the multiple-choice discrimination task challenges.
It is intriguing to speculate that this dissociation between ventral-lesioned rats' ability to withhold responding to PRF cues and other indicators of their sensitivity to intermittent reinforcement may have parallels in simple extinction. Although extinction procedures have been observed to directly depotentiate excitatory amygdala pathways involved in conditioning, reversing changes acquired in the initial acquisition of conditioning (e.g., Kim et al., 2007), considerable evidence also implicates the acquisition of separate inhibitory learning in extinction. For example, Quirk and colleagues (e.g., Peters, Kalivas, & Quirk, 2009) have suggested that IL and DP activity promotes extinction of both conditioned fear and cocaine-seeking behavior by inhibiting the normal excitatory action of amygdala circuitry. Notably, previously extinguished responses recover if this inhibitory input, via projections to GABAergic neurons in the intercalated neuron groups and the lateral division of CeA, is removed. Similarly, lesions of IL have been found to enhance spontaneous recovery, reinstatement, and renewal of extinguished responding (Rhodes & Killcross, 2004, 2007a), all phenomena that reflect a failure to inhibit previously learned responses to a trained and extinguished cue. Our ventral-lesioned rats' impairment in withholding responding to PRF cues may reflect a similar deficit in the face of both excitatory and inhibitory associations to the same cue. Notably, Rhodes and Killcross (2007b) found that after conditioned inhibition training, rats with IL lesions failed to show the normal retardation of learning observed when that inhibitor was retrained as an excitor, but displayed normal evidence for conditioned inhibition in a summation test, in which the inhibitor was presented in compound with another excitor. Recall that in our combined cue test, we observed normal distribution of responding in ventral-lesioned rats when the PRF cue was combined with a CRF cue, analogous to Rhodes and Killcross's (2007b) summation test compound. On the other hand, using a serial feature negative “occasion-settting” (Holland, 1983) procedure, MacLeod and Bucci (2010) found that PL, but not IL, lesions affected rats' ability to respond to a tone when it was presented alone but not when it was preceded by another cue. Given that both our dorsal- and ventral-lesioned rats had substantial damage to IL (and to some extent, PL), it is intriguing to speculate that both IL and PL normally work in concert with more ventral and more dorsal mPFC regions in modulating responding to cues with ambiguous reinforcement history. More generally, it is important to recognize that nonreinforcement of previously reinforced cues (as in extinction and in PRF) may have multiple consequences, including representation of the reinforcement contingencies themselves, inhibition of previously-learned behavior in output (e.g., Li, Nair & Quirk, 2009), and other processes. Different mPFC subregions may work within a variety of circuits in implementing these functions.
By contrast, in our study, rats with dorsal mPFC lesions showed essentially the opposite patterns of results from those we observed in rats with ventral lesions. These rats failed to show the normal pattern of enhanced cue associability after PRF training. Instead, in dorsal-lesioned rats, PRF cues were significantly less able to overshadow conditioning to auditory cues than CRF cues. It is notable that this abnormal allocation of attention to CRF and PRF cues in a new learning task (as measured by interference with tone conditioning) occurred despite normal allocation of responding to CRF and PRF cues in tests both before and after the overshadowing test, both when those cues were presented separately and when they were presented simultaneously. Thus, the dorsal-lesioned rats' inability to use cues' predictive relations with reinforcement to alter their associability did not reflect an overall insensitivity to reinforcement contingencies.
Interestingly, although the observation of greater overshadowing by CRF than PRF cues in dorsal-lesioned rats is counter to results obtained with intact rats (Maddux et al., 2007) and with predictions of the Pearce and Hall (1980) model, it is in fact predicted by other learning theories (Mackintosh, 1975; Rescorla & Wagner, 1972). For example, in Mackintosh's (1975) theory, with individual cue presentations, more consistent predictors of reinforcement garner attention more rapidly, and thus enter into new associations more readily, than less consistent predictors. As a result, in the present study, the CRF port light should overshadow conditioning to the tone more than the PRF port light. Similarly, within the Rescorla-Wagner (1972) model, CRF cues should acquire greater associative strength than PRF cues trained with the same number of trials (but half as many reinforcers). Consequently, according to this model, which likens overshadowing to blocking, CRF cues should therefore produce substantially greater overshadowing or blocking of the tone cues. Thus, it is tempting to suggest that when mechanisms of associability change specified by the Pearce-Hall (1980) model are made unavailable by dorsal mPFC lesions, rats revert to default modes of processing specified by the Rescorla-Wagner (1972) or Mackintosh (1975) theories.
Although there was considerable overlap in the brain damage observed in rats with dorsal or ventral lesions, those lesions had very different effects on several aspects of behavior. Both lesion groups sustained heavy damage to IL and at least partial damage to PL, but for the dorsal-lesioned rats, damage extended dorsally to ACC, and for the ventral-lesioned rats, damage extended ventrally to DP and TT. Because only the ventral-lesioned rats showed an altered distribution of responding to CRF and PRF cues, the ventral-most mPFC regions are likely to be critical to that distribution. However, this deficit could be due either to damage to this extreme ventral region alone, or to the combined damage of that region and IL and PL. Similarly, Maddux et al. (2007) found that in rats that showed comparable abnormalities in the distribution of responding to CRF and PRF cues, cholinergic depletion in the mPFC was most evident in PL and IL but also extended ventrally to the DP and TT (and dorsally to ACC). Thus, whether TT normally functions alone in this task, or in concert with more dorsal portions of mPFC, cannot be determined in the present experiment alone. However, it is notable that Maddux and Holland (2010) found a similar lack of differentiation between responding to individually presented CRF and PRF cues in rats with ventral lesions that included TT, DP, and IL damage but left PL largely intact. Nevertheless, the role of DP and/or TT in altering this (but not other) aspects of sensitivity to reinforcement contingency information remains enigmatic.
Notably, TT is not generally considered part of prefrontal cortex, despite its location in that region of the brain. Rather, the dorsal TT has been conceived to be part of the hippocampal continuation and ventral TT as part of olfactory cortex (Wyss & Sripanidkulchai, 1983), although agreement on this classification is not unanimous (Crosby & Schnitzlein, 1982). Nevertheless, it is interesting to note that whereas most prefrontal regions project broadly to a number of amygdala subnuclei, the TT uniquely project to CeA, a region critical to performance in both 5CSRT and overshadowing tasks used in the present study (Maddux et al., 2007), and do not innervate the basolateral amygdala (Cassell & Wright, 1986; Ottersen, 1982) . Thus, TT might be a reasonable new region of interest for future studies of attentional processes in associative learning.
In a similar vein, because only the dorsal-lesioned rats showed reduced rather than enhanced overshadowing by PRF compared to CRF cues, the dorsal-most regions of mPFC are most likely to be critical to surprise-induced enhancement/maintenance of cue associability. Again, however, it remains to be seen whether ACC mediates this function alone among mPFC subregions, or in tandem with PL and/or IL. Notably, ACC has often been assigned important roles in attention and executive control in studies of both humans (e.g., Kastner & Ungerleider, 2000; Botvinick, Cohen, & Carter, 2004) and animals (e.g., Muir et al., 1996; Passetti et al., 2002), including acting in concert with PPC (e.g., Liston et al., 2006; Nelson, Sarter & Bruno, 2005). As noted earlier, PPC function has been found to be critical to performance in the overshadowing task used here (Maddux et al., 2007), and in related tasks used to assess attention in associative learning (Bucci et al., 1998).
On a broad scale, the results of this study require us to qualify our previous (Maddux et al., 2007) suggestion of a dichotomy between “attention for action” in mPFC and a PPC-based modulation of “attention for learning” in a Pearce-Hall (1980) manner. That dichotomy was indicated by the effects of lesions that removed the cholinergic innervation of most of the mPFC, including in at least some cases, ACC. Those lesions had no effect on the relative abilities of CRF and PRF cues to overshadow conditioning to tones in a task identical to the present one. By contrast, in the present study, neurotoxic dorsal lesions of mPFC reversed enhancements of cue associability normally observed with PRF. It seems reasonable to speculate that mPFC neurons (left intact in Maddux et al.'s, 2007 study) or noncholinergic innervation of those neurons may be critical for the enhanced associability of PRF cues. For example, the mPFC receives strong dopaminergic projections that arise from the midbrain substantia nigra and ventral tegmental areas (Beckstead, Domesick, & Nauta, 1979; Berger, et al., 1974, 1976; Lindvall, Björklund, & Divac, 1978; Simon, Le Moal, & Calas, 1979; Swanson, 1982; Van Eden et al., 1987). These midbrain dopamine cells have been shown to code prediction error in reward learning (Schultz, 1998; Schultz, Dayan, & Montague, 1997), a crucial factor in most associative learning theories (Pearce & Hall, 1980; Rescorla & Wagner, 1972). Furthermore, evidence for involvement of the midbrain dopamine system in surprise-induced enhancements in attention already exists. Lee et al. (2006, 2008) showed that such changes in associability were absent in rats with lesions that disconnected dopmaine neurons in the the substantia nigra pars compacta from CeA. Interestingly, not only does the mPFC receive midbrain dopaminergic input, but also it sends projections to CeA (Buchanan et al., 1994; Hurley et al., 1991; McDonald et al., 1996). Thus, the mPFC is well-situated to participate in this neural circuit subserving incremental attentional processes. Additional research is needed to clarify how dorsal mPFC works together with circuitry previously identified as critical to such changes in attention for new associative learning.
Finally, our results support a dorsal-ventral distinction within the mPFC in the use of reinforcement prediction information in the allocation of attention in new learning and in the control of action to individually presented cues. Although this distinction bears little resemblance to other dorsal-ventral distinctions that have been made for mPFC function, for example those of place vs. object (e.g., Levy & Goldman-Rakic, 2000), movement vs. choice (Passingham & Toni, 2001), action vs. object identification (Milner & Goodale, 1995) or production vs. inhibition (Peters et al., 2009), it may lead to a better understanding of how attentional processes modulate associative learning.
Acknowledgments
Funding: This work was supported by the National Institutes of Health (grant number MH53367).
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Beckstead RM, Domesick VB, Nauta WJ. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Research. 1979;175:191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- Berger B, Tassin JP, Blanc G, Moyne MA, Thierry AM. Histochemical confirmation for dopaminergic innervation of the rat cerebral cortex after destruction of the noradrenergic ascending pathways. Brain Research. 1974;81:332–337. doi: 10.1016/0006-8993(74)90948-2. [DOI] [PubMed] [Google Scholar]
- Berger B, Thierry AM, Tassin JP, Moyne MA. Dopaminergic innervation of the rat prefrontal cortex: a fluorescence histochemical study. Brain Research. 1976;106:133–145. doi: 10.1016/0006-8993(76)90078-0. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. Journal of Neuroscience. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Buchanan SL, Thompson RH, Maxwell BL, Powell DA. Efferent connections of the medial prefrontal cortex in the rabbit. Experimental Brain Research. 1994;100:469–483. doi: 10.1007/BF02738406. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Holland PC, Gallagher M. Removal of cholinergic input to rat posterior parietal cortex disrupts incremental processing of conditioned stimuli. Journal of Neuroscience. 1998;18:8038–8046. doi: 10.1523/JNEUROSCI.18-19-08038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell PJ. Behavioral approaches to the assessment of attention in animals. Psychopharmacology. 1998;138:231–259. doi: 10.1007/s002130050668. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Wright DJ. Topography of projections from the medial prefrontal cortex to the amygdala in the rat. Brain Research Bulletin. 1986;17:321–333. doi: 10.1016/0361-9230(86)90237-6. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behavioural Brain Research. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Crosby EC, Schnitzlein HN. Comparative correlative neuroanatomy of the vertebrate telencephalon. Macmillan; New York: 1982. [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neuroscience and Biobehavioral Reviews. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. Journal of Comparative Neurology. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kim J, Lee S, Park K, Hong I, Song B, Son G, Park H, Kim WR, Park E, Choe HK, Kim H, Lee C, Sun W, Kim K, Shin KS, Choi S. Amygdala depotentiation and fear extinction. Proceedings of the National Academy of Sciences. 2007;104:20955–20960. doi: 10.1073/pnas.0710548105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pelley ME. The role of associative history in models of associative learning: A selective review and a hybrid model. Quarterly Journal of Experimental Psychology: Comparative and Physiological Psychology. 2004;57(B):193–243. doi: 10.1080/02724990344000141. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Youn JM, Gallagher M, Holland PC. Temporally-limited role of substantia nigra-central amygdala connections in surprise-induced enhancement of learning. European Journal of Neuroscience. 2008;27:3043–3049. doi: 10.1111/j.1460-9568.2008.06272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Youn JM, O MJ, Gallagher M, Holland PC. Role of substantia nigra-amygdala connections in surprise-induced enhancement of attention. Journal of Neuroscience. 2006;26:6077–6081. doi: 10.1523/JNEUROSCI.1316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Experimental Brain Research. 2000;133:23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- Li G, Nair SS, Quirk GJ. A biologically realistic network model of acquisition and extinction of conditioned fear associations in lateral amygdala neurons. Journal of Neurophysiology. 2009;101:1629–1646. doi: 10.1152/jn.90765.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Björklund A, Divac I. Organization of catecholamine neurons projecting to the frontal cortex in the rat. Brain Research. 1978;142:1–24. doi: 10.1016/0006-8993(78)90173-7. [DOI] [PubMed] [Google Scholar]
- Liston C, Matalon S, Hare TA, Davidson MC, Casey BJ. Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task-switching paradigm. Neuron. 2006;50:643–653. doi: 10.1016/j.neuron.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. A theory of attention, variations in the associability of stimuli with reinforcement. Psychological Review. 1975;82:276–298. [Google Scholar]
- MacLeod JE, Bucci DJ. Contributions of the subregions of the medial prefrontal cortex to negative occasion-setting. Behavioral Neuroscience. 2010;124:321–328. doi: 10.1037/a0019344. [DOI] [PubMed] [Google Scholar]
- Maddux JM, Holland PC. Effects of dorsal or ventral medial prefrontal cortical lesions on five-choice serial reaction time performance in rats. 2010 doi: 10.1016/j.bbr.2011.02.031. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddux JM, Kerfoot EC, Chatterjee S, Holland PC. Dissociation of attention in learning and action: effects of lesions of the amygdala central nucleus, medial prefrontal cortex, and posterior parietal cortex. Behavioral Neuroscience. 2007;121:63–79. doi: 10.1037/0735-7044.121.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behavioral Neuroscience. 1996;110:247–265. doi: 10.1037//0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Ross RS, Eichenbaum H. Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience. 2008;153:63–71. doi: 10.1016/j.neuroscience.2008.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner AD, Goodale MA. The visual brain in action. Oxford University Press; Oxford: 1995. [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. The cerebral cortex of the rat and visual attentional function, dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cerebral Cortex. 1996;6:470–481. doi: 10.1093/cercor/6.3.470. [DOI] [PubMed] [Google Scholar]
- Murphy ER, Dalley JW, Robbins TW. Local glutamate receptor antagonism in the rat prefrontal cortex disrupts response inhibition in a visuospatial attentional task. Psychopharmacology. 2005;179:99–107. doi: 10.1007/s00213-004-2068-3. [DOI] [PubMed] [Google Scholar]
- Nelson CL, Sarter M, Bruno JP. Prefrontal cortical modulation of acetylcholine release in posterior parietal cortex. Neuroscience. 2005;132:347–359. doi: 10.1016/j.neuroscience.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Ottersen OP. Connections of the amygdala of the rat IV: corticoamygdaloid and intraamygdaloid connections as studied with axonal transport of horseradish peroxidase. Journal of Comparative Neurology. 1982;205:30–48. doi: 10.1002/cne.902050104. [DOI] [PubMed] [Google Scholar]
- Passetti F, Chudasama Y, Robbins TW. The frontal cortex of the rat and visual attentional performance: dissociable functions of distinct medial prefrontal subregions. Cerebral Cortex. 2002;12:1254–1268. doi: 10.1093/cercor/12.12.1254. [DOI] [PubMed] [Google Scholar]
- Passingham RE, Toni I. Contrasting the dorsal and ventral visual systems: guidance of movement versus decision making. Neuroimage. 2001;14:S125–131. doi: 10.1006/nimg.2001.0836. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd ed. Academic Press; San Diego, CA: 1997. [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning, variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychological Review. 1980;87:532–552. [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learning & Memory. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black A, Prokasy WF, editors. Classical Conditioning II: Current research and theory. Appleton-Century-Crofts; New York: 1972. pp. 64–99. [Google Scholar]
- Rhodes SEV, Killcross AS. Lesions of rat infralimbic cortex enhance recovery and reinstatement of an appetitive Pavlovian response. Learning and Memory. 2004;11:611–616. doi: 10.1101/lm.79704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes SEV, Killcross AS. Lesions of rat infralimbic cortex enhance renewal of extinguished appetitive Pavlovian responding. European Journal of Neuroscience. 2007a;25:2498–2503. doi: 10.1111/j.1460-9568.2007.05486.x. [DOI] [PubMed] [Google Scholar]
- Rhodes SEV, Killcross AS. Lesions of rat infralimbic cortex result in disrupted retardation but normal summation test performance following training on a Pavlovian conditioned inhibition procedure. European Journal of Neuroscience. 2007b;26:2654–2660. doi: 10.1111/j.1460-9568.2007.05855.x. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Simon H, Le Moal M, Calas A. Efferents and afferents of the ventral tegmental-A10 region studied after local injection of [3H]leucine and horseradish peroxidase. Brain Research. 1979;178:17–40. doi: 10.1016/0006-8993(79)90085-4. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Research Bulletin. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Van Eden CG, Hoorneman EM, Buijs RM, Matthijssen MA, Geffard M, Uylings HB. Immunocytochemical localization of dopamine in the prefrontal cortex of the rat at the light and electron microscopical level. Neuroscience. 1987;22:849–862. doi: 10.1016/0306-4522(87)92964-2. [DOI] [PubMed] [Google Scholar]
- Wyss JM, Sripanidkulchai K. The indusium griseum and anterior hippocampal continuation in the rat. Journal of Comparative Neurology. 219:251–272. doi: 10.1002/cne.902190302. [DOI] [PubMed] [Google Scholar]