Abstract
Estrogens have been shown to have a strong influence on such cognitive domains as spatial memory, response learning, and several tasks of executive function, including both working memory and attention. However, the effects of estrogens on inhibitory control and timing behavior, both important aspects of executive function, have received relatively little attention. We examined the effects of estradiol on inhibitory control and timing using a differential reinforcement of low rates of responding (DRL) task. Ovariectomized young (3 month), middle-aged (12 month), and old (18 month) Long-Evans rats received 5% or 10% 17β-estradiol in cholesterol vehicle or cholesterol vehicle alone via Silastic implants and were tested on a DRL task requiring them to wait 15 seconds between lever presses to receive a food reinforcer. The ratio of reinforced to non-reinforced lever presses did not differ across age in the cholesterol vehicle group. Conversely, 17β-estradiol impaired learning of the DRL task in young and middle-aged rats, but the learning of old rats was not impaired relative to vehicle controls following either 5% or 10% 17β-estradiol treatment. Overall, old rats also made fewer lever presses than both the young and middle-aged rats. These results provide new evidence that estrogens impair inhibitory control, an important aspect of self regulation, and add to existing evidence that estrogens differentially affect cognition at different ages.
Keywords: estrogens, inhibition, aging, DRL, timing, operant
1. Introduction1
Extensive research in rodents suggests a strong influence of estrogens on such cognitive domains as working memory and place learning (see [16,24,37]). In contrast, relatively little is known about the effects of estrogens on behavioral inhibition. Behavioral inhibition consists of several related phenomena including the ability to withhold a response and the ability to estimate time [19], and is one of a subset of cognitive processes commonly referred to as executive functions [49].
In animal models behavioral inhibition is often assessed using operant tasks in which the animal must “withhold” a behavioral response for a specific period of time to earn a reinforcer [18,20,50,65,76]. Of these, the differential reinforcement of low rates (DRL) of responding operant schedule has proven to be a sensitive task to measure the ability to delay previously reinforced behavior [2,20,50]. In the operant DRL, a response (i.e. a lever press) that results in reinforcement must be withheld for a fixed period of time before that response will again be reinforced [64,76]. Premature responses that occur during this fixed period are not reinforced and result in a “resetting” of the wait period.
Research addressing the effects of estrogens on the ability to learn DRL tasks is sparse and conflicting [4,40,76]. In one study [4], ovariectomy (OVX) was found to impair the ability of young rats (5 months) to learn a DRL task as compared to intact controls. Specifically, OVX rats had a lower efficiency ratio (reinforced presses/total presses), earned fewer reinforcers, and made more lever presses than intact controls [4]. However, Lentz [40] citing a personal communication from Beatty (the author of [4]) described the findings of a follow-up study in which Beatty failed to replicate the deleterious effect of OVX on DRL learning (see discussion in [40]). Lentz and colleagues [40] also failed to uncover an OVX-induced difference between young (6 months) adult OVX rats and intact control rats on a similar DRL task [40]. Lentz and colleagues [40] further found that chronic treatment of OVX rats with a supraphysiological level of estradiol (10 ng/ml in the serum; typical physiological levels in rodents range from 3-90 pg/ml [57]) failed to produce any effect on DRL efficiency.
We recently completed a study in which OVX young adult rats (6 months) treated chronically with a physiological level of estradiol (86 pg/ml in the serum) performed worse than did cholesterol vehicle controls during acquisition of a DRL task [76]. Estradiol-treated rats made more lever presses and had a lower efficiency than both OVX cholesterol controls and intact rats. In agreement with Lentz et al. [40], performance did not differ between OVX cholesterol controls and intact rats. Our results are also consistent with a recent study showing hormone effects on timing estimates in a peak-interval task [66]. In that study, OVX adult female rats (3 months) were initially trained on a fixed-interval procedure in which rats were free to lever press at any time, but a lever press was only associated with reinforcement either 7- or 21-seconds after the illumination of a cue light. Rats were then exposed to the peak-interval procedure, in which they were again free to respond at any time following the onset of the cue light, but only half of the presses occurring after the target interval were associated with reinforcement. On the non-reinforced trials the lever remained extended for a duration 2.5-3 times the target interval, allowing assessment of the peak interval for responding. Although both DRL and peak-interval tasks assess timing behavior, there is no “resetting” of the time interval associated with peak-interval tasks. The animal is free to respond at any time without negative consequence and peak time of responding is dissociable from overall response rate [63]. As such, peak time tasks allow measurement of both over- or undershooting of the target time interval. Following acquisition of the peak-interval task, rats were exposed to estradiol treatment (proestrus levels: peak serum levels of 88 pg/ml) and again exposed to the peak-interval procedure. Estradiol produced a leftward shift in the peak response time for both 7- and 21-second target intervals, resulting in an undershooting of both target intervals [66], suggestive of an increase in the speed of the internal clock [46,63,66].
DRL tasks are sensitive not only to hormone status but also to age at testing. However, it is important to point out that the effects of aging have only been assessed in male rats. Aged male rats (24-25 months) showed lower response rates and a higher efficiency ratio than did young adult rats (3- or 7- months old) when trained and tested on DRL tasks with intervals ranging from 5-20 seconds [38,70]. These age-related differences in learning were most clearly seen during initial testing sessions of both DRL-5 [70] and DRL-20 [38] schedules. With extended testing these differences diminished [38,70]. Performance of a peak-interval task was also found to be affected by age at testing, with aged male rats (24-26 months) showing a rightward shift in peak response time based upon a 20-second target interval, resulting in aged animals overshooting the target interval (e.g., underestimating the passage of time, [46,63]) in comparison with younger rats (4-6 months) [39,48].
Although these studies were conducted with male rats, these findings do suggest that an effect of estradiol on DRL acquisition could be moderated by age at testing in female rats. Therefore, a goal of this study was to expand our previous finding that chronic estradiol treatment impaired learning of a DRL task in young female rats to determine whether different results would be obtained in old rats. In addition, because little is known about the effects of estradiol on DRL acquisition at middle-age, a time during which significant changes in reproductive status, associated hormonal levels, and cognition begin to occur [45,82], we also included middle-aged (approximately 14-months old at time of testing) rats in the study.
2. Methods
2.1 Animals and exposure
A total of 144 female Long-Evans rats were obtained from Harlan (Indianapolis, IN), and were housed in a temperature and humidity controlled room (22°C, 40-55% humidity) under a 12-hour reverse light-dark cycle(lights off 8:30 am). All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Illinois at Urbana-Champaign and were in accordance with the guidelines of the Public Health Service Policy on Humane Care and Use of Laboratory Animals [51] and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research [52].
The rats were received in two cohorts of 72 animals each, separated by 6 months. Due to the extensive number of rats needed to complete this study and the limits of available operant testing chambers (see below), it was necessary to conduct the study in two cohorts balanced for age and estradiol treatment. Each cohort consisted of 24 young (3 month old) virgin females, 24 middle-aged (12 month old) retired breeders and 24 old (18 month old)retired breeders. Each age group was divided into three estradiol treatment groups (cholesterol vehicle, 5% estradiol, and 10% estradiol) [42] with 8 rats per age per dose in each of the two cohorts, or a total of 16 rats in each dose group at each age. Rats from the same treatment groups were pair-housed in standard polycarbonate cages (45 × 24 × 20 cm) with corncob bedding.
After a one-week period of acclimation to the vivarium, rats were OVX and a Silastic capsule containing 5% or 10% 17β-estradiol in cholesterol (Sigma, St. Louis, MO) or cholesterol vehicle alone (as described in [42]) was implanted subcutaneously at the nape of the neck under isoflurane gas anesthesia (VetEquip, Pleasanton, CA). One end of the Silastic capsule (1.5 mm i.d., 1.96 mm o.d.) was plugged with 0.25 cm silicone and dried overnight before packing with 1 cm of the estradiol/cholesterol mixture or cholesterol vehicle after which the other end was plugged with 0.25 cm silicone. Capsules were soaked in sterile saline at 37°C overnight before insertion during surgery.
The diet was switched from standard rat chow to AIN-93G on the day of OVX surgery. This was done to ensure that the rats were not exposed to additional estrogens via the diet, which becomes increasingly important for long-term behavioral studies. Research has shown that the amount of soy phytoestrogens in standard lab chow can vary greatly, even across different lot numbers from the same supplier [8,73,74]. Beginning one week after surgery, rats were food restricted to and maintained at 85% of their free-feeding body weights. The average weight in grams (Mean±SEM) following restriction to 85% for young rats was 223.42±2.82, for middle-aged rats was 279.06±3.80, and for old rats was 287.22±5.71. After the rats had been food restricted for one week they were tested for a single day on a dual solution T-maze task that tests for biases in learning strategy. Rats were trained to collect a food reinforcer located in one arm of a T-maze (goal arm). The start and goal arms were held constant with respect to extra-maze cues. After training to criterion, the rats were given a single probe trial in which the start arm was rotated 180° from the position used during training. Rats were assumed to be using a “place”strategy if they entered the arm in the spatial location that was correct during training, while a response strategy was indicated if the rat made a turn in the same direction that was correct during training. Although previous studies using shorter term higher dose estradiol treatment produced a shift towards a place strategy to solve this task [36], we failed to uncover any differences in the response strategies used by 17β-estradiol-and the cholesterol vehicle-treated rats (data not reported). Operant training began the day following T-maze testing. The rats were tested six days per week (Monday through Saturday) during the dark phase of the light cycle.
All rats were subject to response shaping and lever press training (as described below) and were then tested on a working memory task, delayed spatial alternation (DSA) [77], which took approximately 2 months to complete, before beginning DRL training. At the beginning of DRL training, the young group was approximately 5 months old, the middle-aged group was approximately 14 months old, and the old group was approximately 20 months old.
2.2 Operant testing
Behavioral testing was conducted in 16 standard automated operant chambers (Med-Associates Inc., St. Albans, VT) housed in sound-attenuated wooden boxes (interior dimensions: 55.9 cm wide, 38.1 cm high, 35.6 cm deep). All of the test chambers had the same features and dimensions: 21.6 cm tall, with a 29.2 cm wide and 24.8 cm deep stainless-steel grid floor resting just above a tray filled with corn cob bedding. A pellet trough was centered 2.5 cm above the floor on the operant panel. Positioned symmetrically on both sides of the pellet dispenser were a pair of retractable response levers and a pair of stimulus cue lamps, one above each lever. The levers were 5.7 cm from the midline and 7.0 cm above the floor and the cue lights (clear, 1.12 watts (28V), 9 mm in diameter) were located 5.7 cm above the levers. Each chamber also contained a Sonalert tone generator, a white noise generator, and a house light located on the back wall. Experimental contingencies were programmed using the Med-State behavioral programming language (Med-Associates, VT). Forty-five mg soy-free purified rodent diet food pellets (Formula P, P.J. Noyes Inc., Lancaster, NH) were used as reinforcers in the study.
Rats were tested in the appetitively reinforced operant DRL test and were fed a limited amount of food 30 minutes after behavioral testing was completed each day. The total amount of food provided was sufficient to maintain the rats at approximately 85% of their free-feeding weights. Most rats consumed the total ration within 2-3 hours and thus were approximately 18-20 hours food deprived when they entered the testing chamber each day. This feeding schedule was necessary to insure that the rats were motivated to work for the food reinforcers used in the cognitive testing.
2.3 Response shaping and lever press training
Rats were trained to press the response levers using an autoshaping program [76,77]. Autoshaping test sessions terminated after 60 minutes elapsed or 100 reinforcers were delivered, whichever occurred first. Criterion for this condition was set at 100 lever presses within a single session. All rats acquired the lever-pressing response within 2-3 sessions. Following autoshaping, the rats were exposed to a continuous reinforcement schedule in which the lever associated with reinforcement was alternated following delivery of every fifth reinforcer. The purpose of this schedule was to strengthen the recently acquired lever press response and to prevent the rats from developing a lever or side preference. This cycle of alternating levers terminated after 100 reinforcers were received or 60 minutes had elapsed. A performance criterion of 100 reinforcers for two consecutive sessions was established for this condition. Following lever press training, the rats were tested on the DSA task [77]. DRL training began on the day immediately following completion of 25 sessions of testing on the DSA task.
2.4 Differential Reinforcement of Low Rates of Responding (DRL)
Timing ability and inhibitory control were assessed using a DRL-15 task. Rats were tested once per day, six days per week. Test sessions were terminated after 90 minutes or 200 reinforcers earned, whichever occurred first. For DRL testing one response lever (left) was used, and the lever remained extended throughout the session. Immediately prior to DRL-15 testing the rats went through three training phases which lasted for 6 sessions. Rats did not have to meet a performance criterion before moving on to the next stage of training. The first two sessions of training consisted of a fixed-ratio 1 (FR-1) schedule in which every lever press resulted in the delivery of a food reinforcer. Sessions 3 and 4 of training consisted of a DRL-5 second schedule in which reinforcement was contingent upon at least a 5 second separation between responses. If a response occurred within the 5-second window, the response timer was reset. Sessions 5 and 6 consisted of a DRL-10 second schedule. The rats were then tested for 30 consecutive sessions on a DRL-15 second schedule (DRL-15). Following completion of the 30 sessions of DRL-15 testing, rats were tested for 3 sessions on an extinction schedule of the DRL-15 task during which lever presses were no longer associated with reinforcement. An extinction schedule was included in order to determine if the rats were sensitive to a shift in reinforcement contingencies.
2.5 Statistical analyses
The behavioral data were analyzed via repeated measures ANOVA using SPSS for Windows, Version 15.0. Treatment, age, and cohort were included in the analyses as between subject factors and significance was set at p<0.05. Marginal significance was set at p>0.05 but <0.10. When appropriate, Tukey post hoc tests were run for pair-wise comparisons.
For DRL-15, the reinforced to non-reinforced lever press ratio and total lever presses were the primary measures of learning. Although an increase in the number of lever presses can be directly related to a decrease in performance efficiency, it is important to note that very low rates of responding will affect efficiency ratios as well, inflating performance efficiency. Therefore, the data were analyzed for both reinforced to non-reinforced ratio and total presses. The data were first averaged to yield six blocks of five test sessions each, and then analyzed using a 3 (treatment) × 3 (age) × 2 (cohort) × 6 (block) mixed ANOVA with block as a repeated measures factor.
Inter-response times (IRTs) representing the delay between lever presses were also examined. IRT distributions provide information as to the temporal location when responses “peak”. IRTs were divided into eight 2.5-sec intervals (e.g., 0<2.5 sec, 2.5<5.0 sec, etc.) with all IRTs longer than 17.5-sec falling in the last IRT interval. The IRT data from the initial DRL-15 testing block and the last DRL-15 testing block were analyzed separately using a 3 (treatment) × 3 (age) × 2 (cohort) × 8 (IRT interval) mixed ANOVA with IRT interval (1-8) as a repeated measures factor. This was done to determine the extent of response inhibition at the beginning of testing and to assess whether extended training shifted the pattern of responding to favor longer IRTs by the last block of testing. For the extinction sessions, the total number of responses on each of the three sessions of testing was analyzed using a 3 (treatment) × 3 (age) × 2 (cohort) × 3 (session) ANOVA.
3. Results
3.1 DRL
3.1.1 Reinforced to non-reinforced responses
Both of the between subject’s variables, age (young, middle-aged or old) and estradiol treatment (0, 5 or 10%) influenced the ratio of reinforced to non-reinforced responses, which was a primary measure of performance on this task (Figure 1). Chronic estradiol treatment significantly impaired performance in young and middle-aged rats but not in old rats, an observation that was supported by a significant age × estradiol group interaction, F(4, 117)=2.637, p=0.037. Analyses for simple main effects of hormone treatment at each age showed that there was a significant main effect of estradiol treatment in young rats, F(2, 45)=4.751, p=0.013, and in middle-age rats, F(2, 42)=12.457, p<0.001, but not in the old rats, F(2, 39)=0.672, p=0.516. In young rats, the impairing effect of estradiol was present in the 10% estradiol group, as evidenced by a significant decrease in the ratio of reinforced to non-reinforced responses compared to cholesterol controls (p<0.05) while the 5% estradiol group did not differ significantly from the control group (p>0.05) (Figure 1). In addition, both the middle-aged 5% and 10% estradiol groups had lower ratios of reinforced to non-reinforced responses than did the cholesterol control group (p<0.05) (Figure 1) in this measure. Analyses for simple main effects of age in each estradiol group revealed a significant simple main effect of age in the 10% estradiol group, F(2, 39)=3.505, p=0.04. Specifically, for the 10% group only, the old rats tended to have a higher ratio of reinforced to non-reinforced responses than did the young (p=0.059) or middle-aged groups (p=0.067), although these differences were only marginally significant (Figure 1).
Figure 1.
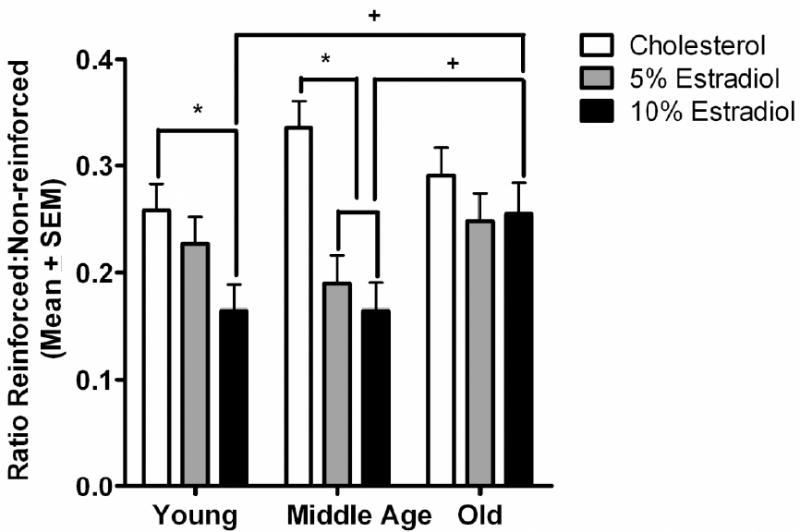
Ratio of reinforced to non-reinforced lever presses at each age averaged across all 30 test sessions of DRL. The young 10% estradiol rats had a lower ratio of reinforced to non -reinforced lever presses than the young cholesterol group (*p<0.05). The middle-aged 5% and 10% estradiol rats had a lower ratio of reinforced to non-reinforced lever presses than the middle-aged cholesterol group (*p<0.05). The old 10% estradiol rats had a marginally significant higher ratio of reinforced to non-reinforced lever presses than both the young 10% estradiol (+p<0.10) and the middle-aged 10% estradiol (+p<0.10) groups.
In addition to the treatment-related effects described above, there was a main effect of cohort, F(1,117)=4.639, p=0.033, with the rats in cohort 2 demonstrating a small, but significantly higher ratio of reinforced to non-reinforced responses than the rats in cohort 1 indicating that, overall, the rats in cohort 2 performed somewhat better on the task, irrespective of treatment group. No other cohort effects and no interactions of cohort with treatment or age were measured during DRL testing.
The ratio of reinforced to non-reinforced responses increased across blocks of testing, indicating that the performance of all treatment and age groups improved over time. This was confirmed by a significant main effect of block, F(5,585)=122.325, p<0.001.
3.1.2 Lever presses
Estradiol treatment and age both influenced the number of lever presses. However, in contrast to the findings for ratio of reinforced to non-reinforced responses, there was not a significant age × estradiol treatment interaction for lever presses. Repeated measures ANOVA revealed both a significant main effect of estradiol treatment, F(2, 117)=11.896, p<0.001, and significant estradiol × block interaction, F(10, 585)=3.158, p=0.008. Tukey post hoc analyses revealed both the 5% and 10% estradiol treated groups to make more presses than the cholesterol control group in testing blocks 1, 2 and 5 (p<0.05: Figure 2A), and the 10% estradiol group to make more presses than the cholesterol control group in testing blocks 3 and 6 (p<0.05: Figure 2A). These effects were reflected in an overall main effect as Tukey post hoc analyses found both the 5% and 10% estradiol groups made more lever presses during DRL than did the cholesterol group, (p<0.05).
Figure 2.
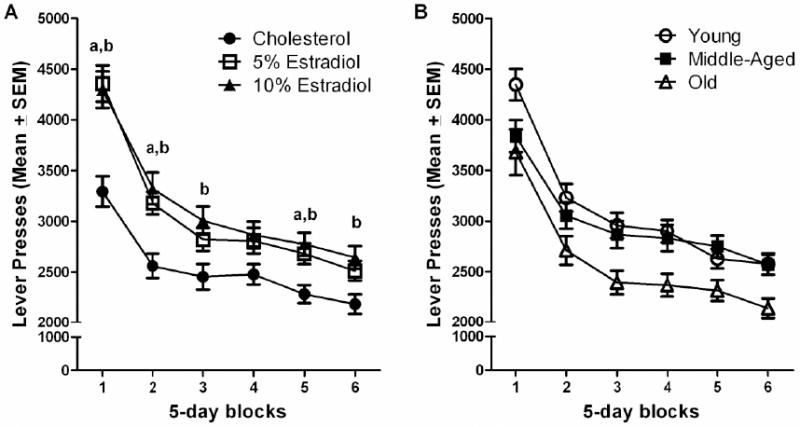
(A) Lever presses across six 5-day blocks of testing for treatment dose. The 5% and 10% estradiol groups made more lever presses than did the cholesterol group in blocks 1, 2 and 5 (a,bp<0.05). The 10% estradiol group made more lever presses than the cholesterol group in blocks 3 and 6 (bp<0.05). (B). Lever presses across six 5-day blocks of testing for age. Overall, the old rats made fewer presses overall than both the young and middle-aged groups (p<0.05).
Although there was not an interaction of estradiol with age, there was a significant main effect of age on lever presses, F(2, 117)=7.333, p=0.001. Tukey post hoc analyses found the old rats to make significantly fewer lever presses overall than did both the young and middle-aged rats, p<0.05 (Figure 2B).
The number of lever presses decreased across blocks of testing in all groups (Fig. 2A and 2B) indicating that all groups improved their performance over time. This was confirmed by a significant main effect of block, F (5, 585)=119.871, p<0.001.
3.1.3. IRT during Initial and Final block for estradiol treatment
No estradiol treatment × age × IRT interaction was uncovered, thus the IRT analyses are reported first for estradiol and then for age. Inter-response times during the first block of testing were sensitive to estradiol treatment. Repeated measures ANOVA revealed a significant IRT bin x estradiol treatment interaction during the first block of DRL testing, F(14, 819)=4.002, p=0.003. Tukey post hoc analyses revealed that the 5% and 10% estradiol groups made significantly fewer responses in the last 3 IRT bins (12.5<15.0 sec, 15.0<17.5 sec and >17.5 sec) than did the cholesterol control group. A similar pattern was observed for the 10.0<12.5 IRT bin but only the 5% estradiol group made significantly fewer responses, p<0.05 (Figure 3A). In contrast, the estradiol groups made more responses in first three IRT bins.
Figure 3.
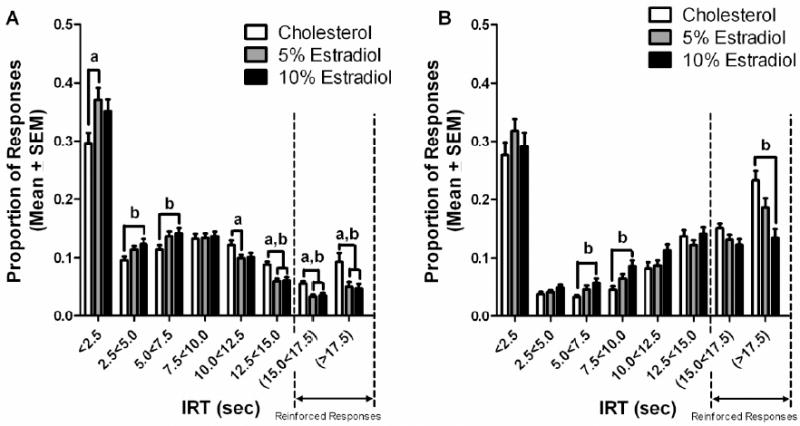
(A) Proportion of total responses in each 2.5 sec inter-response time (IRT) bin during the initial 6 testing sessions for treatment dose. The cholesterol group made more responses during the 12.5<15.0 sec, 15.0<17.5 sec, and >17.5 sec bins than both the 5% and 10% estradiol groups (a,bp<0.05). The 5% estradiol group made more responses than the cholesterol group during the <2.5 sec bin, while making fewer responses than the cholesterol group in the 10.0<12.5 sec IRT bin(ap<0.05). The 10% estradiol group made more responses during the 2.5<5.0 and 5.0<7.5 IRT bins than the cholesterol group bp<0.05). (B) Proportion of total responses in each 2.5 sec IRT bin during the final 6 testing sessions for treatment dose. The cholesterol group made fewer responses than the 10% estradiol group in the 5.0<7.5 sec and 7.5<10.0 sec bins, while making more responses in the >17.5 sec IRT bin (bp<0.05).
For the final block of testing, repeated measures ANOVA uncovered a significant IRT bin × estradiol treatment interaction, F(14, 819)=3.343, p=0.006 (Figure 3B). Tukey post hoc analyses found the 10% estradiol group to make more responses than the cholesterol control group in both the 5.0<7.5 sec and 7.5<10.0 sec IRT bins, bins which are not associated with reinforcement (Figure 4B). Conversely, the 10% estradiol group made fewer responses than the cholesterol control group in the >17.5 sec IRT bin, a bin which is associated with reinforcement, p<0.05 (Figure 4B).
Figure 4.
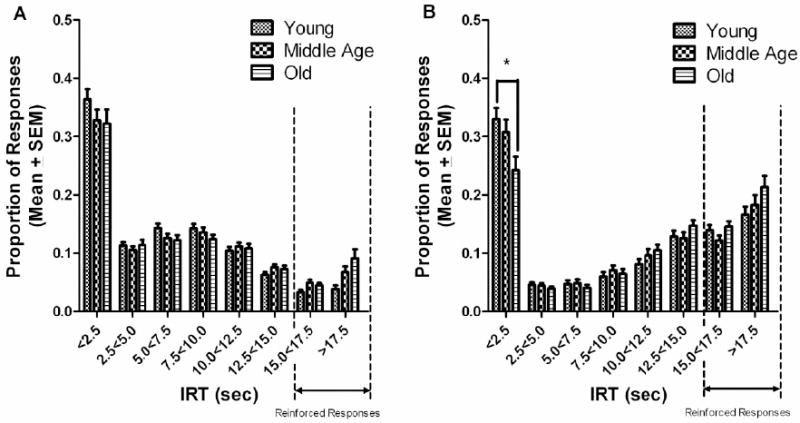
(A) Proportion of total responses in each 2.5 sec inter-response time (IRT) bin during the initial 6 testing sessions for age. (B) Proportion of total responses in each 2.5 sec IRT bin during the final 6 testing sessions for age. The young rats made more responses in the initial bin (<2.5 sec) than did the old age group (*p<0.05).
As Figures 3A and 3B show, all treatment groups exhibited a large amount of ‘bursting’ (defined as responses in the initial <2.5 sec bin) during both the initial and final blocks of the DRL-15 schedule. These early responses are typical of DRL and other operant timing tasks [see 6,34,61].
3.1.4 IRT during Initial and Final block for age
Inter-response times were not influenced by age during the initial block of testing, p>0.05 (Fig. 4A). However, analyses did reveal a significant IRT bin x age interaction for the final block of testing, F(14, 819)=2.669, p=0.022. Tukey post hoc analyses found the young rats to make more responses than did the old rats in the first IRT bin (<2.5 sec), p<0.05 (Figure 4B). As Figures 4A and 4B show, all age groups tended to exhibit a large amount of ‘bursting’ (responses in the <2.5 sec bin) during the initial block of DRL-15 testing: however, the old age group made fewer ‘burst’ responses than the young age group during the last block of DRL-15 testing.
A significant main effect of IRT bin was also uncovered for both the initial block, F(7,819)=228.152, p<0.001, and final block of testing, F(7, 819)=133.044, p<0.001. During the initial block of the task, the number of responses per IRT bin decreased as the IRT bin became longer. However, in contrast to the pattern during the initial block, excluding the first bin, the number of responses (waits) of longer duration increased as the IRT became longer during the final block of testing.
3.2 DRL Extinction
3.2.1 Lever presses during extinction
No estradiol × age × block interaction was uncovered for lever presses made during extinction. However, ANOVA did reveal a significant main effect of estradiol treatment on lever pressing during extinction, F(2, 114)=12.46, p< 0.001. Tukey post hoc analyses found both the 5% and 10% estradiol groups to make significantly more responses during extinction than the cholesterol control group, p<0.05 (Figure 5A). A main effect of age was also observed, F(2, 114)=12.66, p<0.001. Tukey post hoc analyses found the old rats to make significantly fewer responses during extinction than did both the young and the middle-age rats, p<0.05 (Figure 5B).
Figure 5.
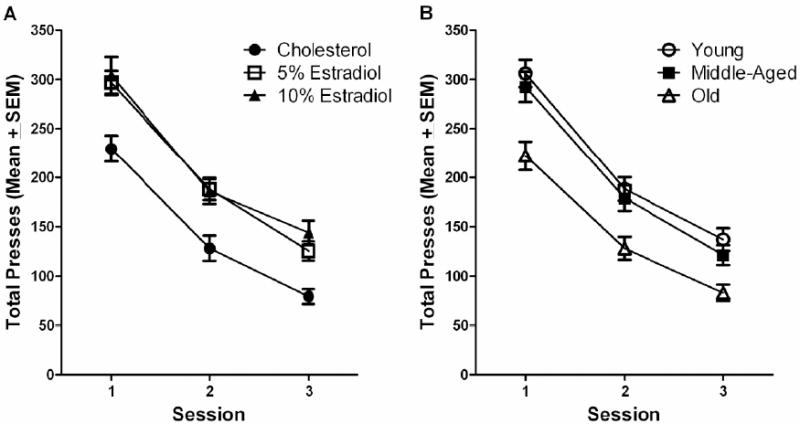
(A) Lever presses across three extinction sessions of the DRL-15 task for treatment dose. The estradiol treated groups made more lever presses overall than the cholesterol control group (p<0.05). (B) Lever presses during across three extinction sessions of the DRL-15 task for age. The young and middle-aged groups made more lever presses overall than the old group (p<0.05).
4. Discussion
We found chronic exposure of rats to estradiol to produce an age-dependent deficit in acquisition of a DRL-15 schedule, an operant task that assesses behavioral inhibition and timing. While estradiol is better known for its enhancing effects on behavior [11,24,42,44], recent findings demonstrate the impairing effects of estradiol on tasks such as response learning, cue learning, conditioned place preference, and some types of working memory tasks [12,14,36,69,76,77,84]. These findings suggest that the effects of estrogens depend upon the type of task and the neural system(s) engaged [16,37].
As seen in Figures 3A and 3B, all treatment groups displayed a bimodal response pattern, emitting many responses during the initial 2.5 sec IRT bin, a behavioral phenomenon that is characteristic of DRL tasks [6,34,61]. However, all groups did learn to delay more of their responses during successive testing sessions, with a second “peak” in responding in the longer IRT bins becoming prominent during the final block of testing (Figure 3b). As the IRT distributions show, the cholesterol-treated rats tended to respond more than the estradiol-treated rats in the latter IRT bins during the initial block of testing, with the highest number of responses shifting to those IRTs during the final block of testing. Conversely, estradiol-treated rats tended to respond more in earlier bins during the initial block of testing, a pattern of responses which continued into the final block of testing. These results reflect the lower efficiency ratio measured in the estradiol treated groups, and indicate that estradiol-treated rats did not acquire this task at the same rate as cholesterol-treated control rats did. These results are also consistent with the leftward shift in peak responding seen on a peak-interval task following estradiol treatment [66].
Few previous studies have addressed the effects of estradiol manipulation on the performance of DRL schedules [4,40,76]. Beatty [4] originally reported that OVX impaired performance on DRL tasks in comparison to sham controls. However, Lentz et al. [40] did not observe a deficit in OVX rats, and, as they discuss, subsequent unpublished research by Beatty also failed to replicate the OVX-induced difference. Lentz et al. [40] also reported that estradiol did not influence DRL performance in OVX rats, a finding that opposed our current and previous [76] results. However, the estradiol doses in the Lentz study [40] led to serum estradiol levels an order of magnitude above typical physiological levels (10 ng/ml vs.3-90 pg/ml [57]), which could be a significant factor in the behavioral differences found in their study and ours. Different estradiol doses have been found to differentially influence behavioral outcomes in other studies [25,30,68,79]. Probably less important, other methodological differences that might have contributed to these opposing findings included ifferences in rat strains [1,41], housing conditions [21,23], and timing of estradiol replacement [13,26,45,76].
4.1 Cohort effects
A significant effect of cohort was found for the ratio of reinforced to non-reinforced lever presses. Overall, the rats tested in the first cohort had, on average, higher efficiency ratios than rats tested in the second cohort. Since the two cohorts were tested 6 months apart, this could be related to seasonal factors. However, there were no interactions of cohort with estradiol treatment or with age that approached statistical significance. Thus, it is unlikely that the cohort differences were the main source of treatment or age effects found in this report.
4.2 Role of activity levels
The DRL schedule provides reinforcement for the withholding of a behavioral response (i.e. a lever press), thus changes in spontaneous activity levels might influence performance on this task. A recent study directly measured activity levels of adult male rats (4-5 months) tested on DRL schedules following treatment with amphetamine [see 62]. Compared to saline-treated controls, amphetamine-treated rats had an increased response rate, a decrease in DRL efficiency, and a leftward shift (undershooting) in the peak of the IRT distribution [22]. Motor behavior measured during testing indicated that amphetamine significantly elevated the amount of locomotion during the ‘wait’ period. In particular, amphetamine-treated rats had more approaches to the operandum (a sensing disc) associated with reinforcement during the ‘wait’ periods [22]. Conversely, saline-treated rats tended to locate themselves away from the operandum and had limited locomotor activity during the ‘wait’ periods. These findings suggest that increased locomotor activity could decrease efficiency on DRL tasks.
4.2.1 Estradiol and activity
Estradiol can influence spontaneous locomotor activity in OVX rats [17,33,56,75]. However, much disparity exists in the outcomes reported following acute or chronic estradiol treatment, ranging from an overall decrease in activity levels to an estradiol induced increase in locomotor activity [17,33,56,75]. Following treatment with Silastic implants of estradiol, activity levels appear to increase for only a short period after implantation (5 days), with no differences in activity level measured following extended exposure (14-35 days) [28,42]. Given that all rats tested in this study had Silastic implants for at least 60 days prior to DRL testing, an estradiol associated increase in activity is unlikely to underlie the observed DRL deficits.
4.2.2. Age and activity
The efficiency of the estradiol-treated old rats in this study was not impaired relative to similarly aged controls. Specifically, the old 10% estradiol treated rats had a higher ratio of reinforced to non-reinforced responses than young or middle-aged 10% estradiol treated rats. Activity levels have been shown to decrease with age in rats [29], and this would be expected to bias aged rats towards performing better in a task that requires withholding a response. In line with this, lower response rates and better efficiency have been reported previously on DRL tasks in aged rats [38,70]. Other studies using antidepressant drugs have found a relationship between a treatment-induced reduction in locomotor activity and reduced response rates during DRL testing [54,55]. Age effects are also evident on the peak-interval task, with aged rats tending to underestimate the passage of time, producing a response peak that exceeds the target interval [36,48]. This age-associated rightward shift in responding to a temporal interval, in combination with an age-associated decrease in response rate, may have countered the estradiol-associated decrement in DRL performance that was observed in young and middle-aged rats. This effect is clearly evident when comparing the performance of old rats to that of middle-aged rats. Specifically, lever pressing was higher in middle-aged cholesterol control rats (Figure 6A) as compared to old cholesterol controls throughout testing (Figure 6B). Although estradiol treatment initially increased the lever pressing rate of old rats, this shift was only evident during the first block of testing. The depressed lever press rate exhibited by old rats appeared to preserve their performance on the DRL task, an effect related to and reflected in this age group s enhanced performance efficiency during DRL-15 testing.
Figure 6.
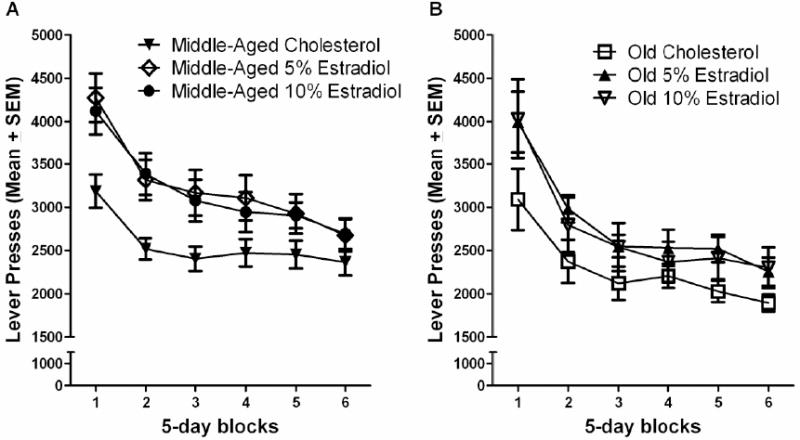
(A) Lever presses across six 5-day blocks of testing for the middle-age group by treatment dose. (B) Lever presses across six 5-day blocks of testing for the old age group by treatment dose.
4.3. Other factors that could account for the age difference in effects
Several other factors could play contributing roles in the age-related differences we observed, including differences in reproductive history and/or age-related changes in estrogen responsiveness. Studies have linked reproductive experience to improved cognitive function, but these effects are often transient in nature or are seen in rats that have given birth to a single litter [58,59]. It is also important to note that the young rats used in this study were nulliparous whereas both the middle-aged and old rats were retired breeders. Importantly, the lack of an estradiol effect was only seen in the old age group, with both the middle-aged (retired breeders) and young (nulliparous) rats being impaired by estradiol treatment. Therefore, the lack of an estradiol effect in old rats does not appear to be related solely to reproductive history.
Another possible explanation for the lack of an effect in older rats is that brain regions mediating response inhibition become less sensitive to estradiol with age. Both the prefrontal cortex and the hippocampus have important roles in response inhibition [9,10,53,67,71,83]. Importantly, the majority of research in rodents has found that both the hippocampus and prefrontal cortex (reviewed in [24,7]) remain responsive to estrogen treatment during aging. Thus, it seems unlikely that old rats were less responsive to estradiol treatment.
4.4 Relationship of DRL deficits to working memory deficits
In this and our previously published report [76], all rats were tested on an operant DSA task prior to DRL testing (see methods section). While it seems unlikely that prior testing on a working memory task would differentially affect response inhibition in control-and estradiol-treated rats, we cannot rule out the possibility that this sequential testing could have led to results different from those that would have been obtained had the rats been naïve at the beginning of DRL training, particularly since estradiol produced deficits in DSA performance [76,77].
The cholesterol control rats tested here also outperformed their estradiol counterparts on the DSA task prior to DRL testing [77]. Specifically, young, middle-aged, and old rats treated with either 5% or 10% estradiol had a lower proportion correct throughout the 25 sessions of DSA testing. Given that deficits in working memory have been linked to disrupted behavioral inhibition (see [3] for a review), it remains a possibility that impaired behavioral inhibition may underlie the deficits on both the DSA and DRL tasks. Rodent models suggest that impulsive-type behaviors (or inability to delay a response) can disrupt performance on working memory tasks [5,15, 60].
Alternatively, deficits in working memory could explain the performance deficits measured on the DRL task. As described by Golman-Rakic [27], the inability to suppress a response could be related to an impairment of working memory. Specifically, in the absence of proper regulation via ‘working memory’, the animal may display an over-learned response (i.e. a lever press), inevitably leading to perseverative behavior and disinhibition [27]. Taken further, rather than a specific ‘timing’ deficit, an inability to ‘remember’ the temporal delay associated with reward, or a suppression in the learning of this temporal discrimination may have contributed to the decreased efficiency measure in the estradiol treated groups [e.g. 31,47,72]. In summary, because of the complex interactions of working memory, timing, and response inhibition, it is difficult to elucidate the specific mechanisms that produced the estradiol-related performance deficits seen on the DRL task.
4.5 Estradiol levels
Due to problems with the RIA kit used in this study, we were not able to obtain reliable measures of serum estradiol levels. However, a recent study, which used Silastic capsules of the same dimensions and specifications as our own, found that the 5% and 10% 17β-estradiol/cholesterol mixtures produced stable serum levels of about 20 pg/ml and 40 pg/ml, respectively, 24 days after implant [43]. Importantly, the implants were found to have released only about 11% of the mixture after 24 days. Therefore, we believe that the Silastic implants we used were still releasing physiological levels of estradiol [57] throughout DRL testing which began about 60 days after the implants were inserted.
4.6 Extinction sessions
The higher response rates of the estradiol treated groups persisted into the extinction schedule that followed DRL-15 testing. It is important to note however, that all treatment groups were sensitive to the change in reinforcement contingencies during extinction of the DRL schedule. This was evidenced by a lower response rate during the first session of extinction as compared to the final block of DRL-15 testing, a downward shift which continued through the remaining 2 extinction sessions (Figure 5A). Age also influenced responding during extinction. Old rats made fewer responses during extinction than their younger counterparts (Figure 5B), a behavioral pattern that was also consistent with that during DRL-15 testing. Elevated lever pressing during extinction schedules is suggestive of a deficit in the ability to alter behavior after a change in reinforcement contingencies [see 32], and is indicative of preservation of the lever press response [see 35,78].
4.7 Role of the light/dark cycle
Therats in this study were tested during the dark portion of a reverse light-dark cycle. Time of testing during the light-dark cycle can influence behavioral outcomes differentially by age. Specifically, when maintained on a reverse light-dark cycle, older rats tended to perform better on a Morris water maze task, while the opposite was true for younger rats [80,81]. Old rats also performed better on an operant delayed alternation task when tested in the early part of the dark cycle vs. later in the dark cycle [80,81]. Although our rats were tested during the dark phase of the light-dark cycle, the performance of the old group was consistent with previously published findings for old animals tested on DRL schedules during the light phase of the cycle [38,70], and with findings in 17β-estradiol treated young rats tested during the light phase on a peak interval task [66].
4.8 Summary and conclusions
In summary, this study found that chronic estradiol treatment impaired the ability of young and middle-aged rats during acquisition of a DRL task when compared to OVX cholesterol control rats. Surprisingly, performance on the DRL task did not vary significantly across treatment groups in old rats, likely related to an overall decrease in response rate in old rats, independent of estradiol dose. Old rats continued to respond at a lower rate during extinction. These results are important for several reasons. First, they provide evidence that chronic estrogen replacement can have a negative impact on the ability to inhibit inappropriate responses, an aspect of executive function that is critical for self regulation. To date very few studies have assessed the impact of estrogen replacement on inhibitory control in animal models or in humans. Second, these results contribute to the growing body of evidence that estrogen replacement can have differential effects on cognitive function at different stages of the lifespan. In the future it will be important to gain a better understanding of the mechanisms underlying these age-related differences in sensitivity.
Acknowledgments
This research was supported by National Institute on Aging Grant P01 AG024387 (SLS), and National Science Foundation IOB 0520876 (DLK). Steven Neese and Victor Wang also received support from National Institute of Environmental Health Sciences Grant T32 ES007326.
Footnotes
Abbreviations: Differential reinforcement of low rates of responding (DRL); ovariectomy (OVX)
Conflict of Interest Statement The authors have no potential conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andrews JS, Jansen JH, Linders S, Princen A, Broekkamp CL. Performance of four different rat strains in the autoshaping, two-object discrimination, and swim maze tests of learning and memory. Physiol Behav. 1995;57:785–790. doi: 10.1016/0031-9384(94)00336-x. [DOI] [PubMed] [Google Scholar]
- 2.Arce E, Santisteban C. Impulsivity: a review. Psicothema. 2006;18:213–220. [PubMed] [Google Scholar]
- 3.Barkley RA. Attention-deficit/hyperactivity disorder, self-regulation, and time: toward a more comprehensive theory. J Dev Behav Pediatr. 1997;18:271–279. [PubMed] [Google Scholar]
- 4.Beatty WW. Effects of gonadectomy on sex differences in DRL behavior. Physiol Behav. 1973;10:177–178. doi: 10.1016/0031-9384(73)90108-x. [DOI] [PubMed] [Google Scholar]
- 5.Bizot JC, Thiebot MH. Impulsivity as a confounding factor in certain animal tests of cognitive function. Cogn Brain Res. 1996;3:243–250. doi: 10.1016/0926-6410(96)00010-9. [DOI] [PubMed] [Google Scholar]
- 6.Blough DS. Interresponse time as a function of continuous variables : a new method and some data. J Exp Anal Behav. 1963;6:237–246. doi: 10.1901/jeab.1963.6-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohacek J, Bearl AM, Daniel JM. Long-term ovarian hormone deprivation alters the ability of subsequent oestradiol replacement to regulate choline acetyltransferase protein levels in the hippocampus and prefrontal cortex of middle-aged rats. J Neuroendocrinol. 2008;20:1023–1027. doi: 10.1111/j.1365-2826.2008.01752.x. [DOI] [PubMed] [Google Scholar]
- 8.Brown NM, Setchell KD. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Lab Invest. 2001;81:735–747. doi: 10.1038/labinvest.3780282. [DOI] [PubMed] [Google Scholar]
- 9.Cho YH, Jeantet Y. Differential involvement of prefrontal cortex, striatum, and hippocampus in DRL performance in mice. Neurobiol Learn Mem. 2010;93:85–91. doi: 10.1016/j.nlm.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Costa VCI, Bueno JLO, Xavier GF. Dentate gyrus-selective colchicine lesion and performance in temporal and spatial tasks. Beh Brain Res. 1995;160:286–303. doi: 10.1016/j.bbr.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- 12.Daniel JM, Lee CD. Estrogen replacement in ovariectomized rats affects strategy selection in the Morris water maze. Neurobiol Learn Mem. 2004;82:142–149. doi: 10.1016/j.nlm.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy, but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- 14.Davis DM, Jacobson TK, Aliakbari S, Mizumori SJ. Differential effects of estrogen on hippocampal- and striatal-dependent learning. Neurobiol Learn Mem. 2005;84:132–137. doi: 10.1016/j.nlm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Dellu-Hagedorn F. Relationship between impulsivity, hyperactivity and working memory: a differential analysis in the rat. Behav Brain Funct. 2006;2 doi: 10.1186/1744-9081-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dohanich GP, Korol DL, Shors TJ. Steroids and Cognition. In: Pfaff D, Arnold A, Rubin R, Fahrbach S, Etgen A, editors. Hormones, Brain and Behavior. 2. Academic Press; New York, NY: 2009. pp. 539–576. [Google Scholar]
- 17.Estrada-Camarena E, Vega Rivera NM, Berlanga C, Fernandez-Guasti A. Reduction in the latency of antidepressants by 17 β-estradiol in the forced swimming test. Psychopharmacology. 2008;201:351–360. doi: 10.1007/s00213-008-1291-8. [DOI] [PubMed] [Google Scholar]
- 18.Evenden JL, Ryan CN. The pharmacology of impulsive behavior in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1998;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- 19.Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- 20.Evenden JL. Impulsivity: a discussion of clinical and experimental findings. J Psychopharmacol. 1999;13:180–192. doi: 10.1177/026988119901300211. [DOI] [PubMed] [Google Scholar]
- 21.Ferdman N, Murmu RP, Bock J, Braun K, Leshem M. Weaning age, social isolation, and gender, interact to determine adult explorative and social behavior, and dendritic and spine morphology in prefrontal cortex of rats. Behav Brain Res. 2007;180:174–182. doi: 10.1016/j.bbr.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Fowler SC, Pinkston J, Vorontsova E. Timing and space usage are disrupted by amphetamine in rats maintained on DRL 24-s and DRL 72-s schedules of reinforcement. Psychopharmacology. 2009;204:213–225. doi: 10.1007/s00213-008-1451-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frick KM, Stearns NA, Pan JY, Berger-Sweeney J. Effects of environmental enrichment on spatial memory and neurochemistry in middle-aged mice. Learn Mem. 2003;10:187–198. doi: 10.1101/lm.50703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frick KM. Estrogens and age-related memory decline in rodents: What have we learned and where do we go from here? Horm Behav. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galea LAM, Wide JK, Paine TA, Holmes MM, Ormerod BK, Floresco SB. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects of working memory. Behav Brain Res. 2001;126:115–126. doi: 10.1016/s0166-4328(01)00255-8. [DOI] [PubMed] [Google Scholar]
- 26.Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- 27.Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond BBiol Sci. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- 28.Gulinello M, Lebesgue D, Jover-Mengual T, Zukin RS, Etgen AM. Acute and chronic estradiol treatments reduce memory deficits induced by transient global ischemia in female rats. Horm Behav. 2006;49:246–260. doi: 10.1016/j.yhbeh.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hagen TM, Ingersoll RT, Wehr CM, Lykkesfeldt J, Vinarsky V, Bartholomew JC, Song MH, Ames BN. Acetyl-L-carnitine fed to old rats partially restores mitochondrial function and ambulatory activity. Proc Natl Acad Sci U S A. 1998;95:9562–9566. doi: 10.1073/pnas.95.16.9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmes MM, Wide JK, Galea LA. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behav Neurosci. 2002;116:928–934. doi: 10.1037//0735-7044.116.5.928. [DOI] [PubMed] [Google Scholar]
- 31.Horwood JM, Ripley TL, Stephens DN. Evidence for disrupted NMDA receptor function in tissue plasminogen activator knockout mice. Behav Brain Res. 2004;150:127–138. doi: 10.1016/S0166-4328(03)00248-1. [DOI] [PubMed] [Google Scholar]
- 32.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;14:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 33.Kanyt L, Stolerman P, Chandler CJ, Saigusa T, Pogun S. Influence of sex and female hormones on nicotine-induced changes in locomotor activity in rats. Pharmacol Biochem Beh. 1998;62:179–187. doi: 10.1016/s0091-3057(98)00140-3. [DOI] [PubMed] [Google Scholar]
- 34.Kirkpatrick K, Church RM. Tracking of the expected time to reinforcement in temporal conditioning procedures. Learn Behav. 2003;31:3–21. doi: 10.3758/bf03195967. [DOI] [PubMed] [Google Scholar]
- 35.Kolb B, Nonneman AJ, Singh RK. Double dissociation of spatial impairments and perseveration following selective prefrontal lesions in rats. J Comp Physiol Psychol. 1974;87:772–780. doi: 10.1037/h0036970. [DOI] [PubMed] [Google Scholar]
- 36.Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116:411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- 37.Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem. 2004;82:309–323. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Lejeune H. Long-term memory for DRL: A comparison between weanling, adult and senescent rats. Physiol Behav. 1989;45:321–329. doi: 10.1016/0031-9384(89)90134-0. [DOI] [PubMed] [Google Scholar]
- 39.Lejeune H, Ferrara A, Soffie M, Bronchart M, Wearden JH. Peak procedure performance in young adult and aged rats: acquisition and adapting to a changing performance criterion. QJ Exp Psychol B. 1998;51:193–217. doi: 10.1080/713932681. [DOI] [PubMed] [Google Scholar]
- 40.Lentz FE, Pool GL, Milner JS. Effects of ovariectomy and hormone replacement on DRL behavior in the rat. Physiol Behav. 1978;20:477–480. doi: 10.1016/0031-9384(78)90333-5. [DOI] [PubMed] [Google Scholar]
- 41.Lindner MD, Schallert T. Aging and atropine effects on spatial navigation in the Morris water task. Behav Neurosci. 1988;102:621–634. doi: 10.1037//0735-7044.102.5.621. [DOI] [PubMed] [Google Scholar]
- 42.Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- 43.Mannino CA, South SM, Inturrisi CE, Quinones-Jenab V. Pharmacokinetics and effects of 17beta-estradiol and progesterone implants in ovariectomized rats. J Pain. 2005;6:809–816. doi: 10.1016/j.jpain.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Markham JA, Pych JC, Juraska JM. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the Morris water maze. Horm Behav. 2002;42:284–293. doi: 10.1006/hbeh.2002.1819. [DOI] [PubMed] [Google Scholar]
- 45.Markowska AL, Savonenko AV. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J Neurosci. 2002;22:10985–10995. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meck WH. Neuropharmacology of timing and time perception. Brain Res Cogn Brain Res. 1996;3:227–242. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- 47.Meck WH, Church RM, Olton DS. Hippocampus, time, and memory. Behav Neurosci. 1984;98:3–22. doi: 10.1037//0735-7044.98.1.3. [DOI] [PubMed] [Google Scholar]
- 48.Meck WH. Temporal memory in mature and aged rats is sensitive to choline acetyltransferase inhibition. Brain Res. 2006;1108:168–175. doi: 10.1016/j.brainres.2006.06.047. [DOI] [PubMed] [Google Scholar]
- 49.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 50.Monterosso J, Ainslie G. Beyond discounting: possible models of impulse control. Psychopharmacology. 1999;146:339–347. doi: 10.1007/pl00005480. [DOI] [PubMed] [Google Scholar]
- 51.National Institutes of Health. Public Health Service Policy on Humane Care and Use of Laboratory Animals. NIH/Office of Laboratory Animal Welfare; Rockville, MD: 2002. [Google Scholar]
- 52.National Research Council Institute for Laboratory Animals Research. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. National Academy Press; Washington, D. C: 2003. [PubMed] [Google Scholar]
- 53.Neill DB. Frontal-striatal control of behavioral inhibition in the rat. Brain Res. 1976;105:89–103. doi: 10.1016/0006-8993(76)90925-2. [DOI] [PubMed] [Google Scholar]
- 54.O’Donnell JM. Effects of the beta-2 adrenergic agonist zinterol on DRL behavior and locomotor activity. Psychpharmacology. 1993;113:89–94. doi: 10.1007/BF02244339. [DOI] [PubMed] [Google Scholar]
- 55.O’Donnell JM. Differential sensitivity to the effects of albuterol on locomotor activity and operant behavior. Psychopharmacology. 1993;113:243–249. doi: 10.1007/BF02245705. [DOI] [PubMed] [Google Scholar]
- 56.Ohtani H, Nomoto M, Douchi T. Chronic estrogen treatment replaces striatal dopaminergic function in ovariectomized rats. Brain Res. 2001;900:163–168. doi: 10.1016/s0006-8993(01)02276-4. [DOI] [PubMed] [Google Scholar]
- 57.Overpeck JG, Colson SH, Hohmann JR, Applestine MS, Reilly JF. Concentrations of circulating steroids in normal prepubertal and adult male and female humans, chimpanzees, rhesus monkey, rats, mice, and hamsters: a literature survey. J Toxicol Environ Health. 1978;4:785–803. doi: 10.1080/15287397809529700. [DOI] [PubMed] [Google Scholar]
- 58.Pawluski JL, Vanderbyl BL, Ragan K, Galea LA. First reproductive experience persistently affects spatial reference and working memory in the mother and these effects are not due to pregnancy or ‘mothering’ alone. Behav Brain Res. 2006;175:157–65. doi: 10.1016/j.bbr.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 59.Pawluski JL, Walker SK, Galea LA. Reproductive experience differentially affects spatial reference and working memory performance in the mother. Horm Behav. 2006;49:143–9. doi: 10.1016/j.yhbeh.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 60.Reading PJ, Dunnett SB. Response inhibition on a delayed matching to position task induces by amphetamine, nicotine and age. Psychopharmacology. 1991;104:137–139. doi: 10.1007/BF02244568. [DOI] [PubMed] [Google Scholar]
- 61.Richards JB, Sabo KE, Seiden LS. DRL interresponse-time distributions: quantification by peak deviation analysis. J Exp Anal Beh. 1993;60:361–385. doi: 10.1901/jeab.1993.60-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Richtand NM. Behavioral sensitization, alternative splicing, and d3 dopamine receptor-mediated inhibitory function. Neuropsychopharmacology. 2006;31:2368–2375. doi: 10.1038/sj.npp.1301163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roberts S. J Exp Psychol Anim Behav Process. 1981;7:242–268. [PubMed] [Google Scholar]
- 64.Sable HJK, Eubig PA, Powers BE, Wang VC, Schantz SL. Developmental exposure to PCBs and/or MeHg: effects on a differential reinforcement of low rates (DRL) operant task before and after amphetamine drug challenge. Neurotoxicol Teratol. 2009;31:149–158. doi: 10.1016/j.ntt.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanabria F, Killeen PR. Evidence for impulsivity in the spontaneously hyperactive rat drawn from complementary response-holding tasks. Behav Brain Funct. 2008;4 doi: 10.1186/1744-9081-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sandstrom NJ. Estradiol Modulation of the Speed of an Internal Clock. Behav Neurosci. 2007;121:422–432. doi: 10.1037/0735-7044.121.2.422. [DOI] [PubMed] [Google Scholar]
- 67.Sindon JD, Rawlins JN, Gray JA, Jarrard LE. Selective cytotoxic lesions of the hippocampal formation and DRL performance in rats. Behav Neurosci. 1986;100:320–329. doi: 10.1037//0735-7044.100.3.320. [DOI] [PubMed] [Google Scholar]
- 68.Sinopoli KJ, Floresco SB, Galea LA. Systemic and local administration of estradiol into the prefrontal cortex or hippocampus differentially alters working memory. Neurobiol Learn Mem. 2006;86:293–304. doi: 10.1016/j.nlm.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 69.Snihur AW, Hampson E, Cain DP. Estradiol and corticosterone independently impair spatial navigation in the Morris water maze in adult female rats. Behav Brain Res. 2008;187:56–66. doi: 10.1016/j.bbr.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 70.Soffie M, Lejeune H. Acquisition and long-term retention of a two-lever DRL schedule: comparison between mature and aged rats. Neurobiol Aging. 1991;12:25–30. doi: 10.1016/0197-4580(91)90035-i. [DOI] [PubMed] [Google Scholar]
- 71.Sokolowski JD, Salamone JD. Effects of dopamine depletions in the medial prefrontal cortex on DRL performance and motor activity in the rat. Brain Res. 1994;642:20–28. doi: 10.1016/0006-8993(94)90901-6. [DOI] [PubMed] [Google Scholar]
- 72.Stephens DN, Cole BJ. AMPA antagonists differ from NMDA antagonists in their effects on operant DRL and delayed matching to position tasks. Psychopharmacology. 1996;126:349–359. doi: 10.1007/BF02246455. [DOI] [PubMed] [Google Scholar]
- 73.Thigpen JE, Setchell KD, Saunders HE, Haseman JK, Grant MG, Forsythe DB. Selecting the appropriate rodent diet for endocrine disruptor research and testing studies. I L A R J. 2004;45:410–416. doi: 10.1093/ilar.45.4.401. [DOI] [PubMed] [Google Scholar]
- 74.Thigpen JE, Setchell KD, Padilla-Banks E, Haseman JK, Saunders HE, Caviness GF, Kissling GE, Grant MG, Forsythe DB. Variations in phytoestrogen content between different mill dates of the same diet produces significant differences in the time of vaginal opening in CD-1 mice and F344 rats but not in CD Sprague-Dawley rats. Environ Health Perspect. 2007;115:1717–1726. doi: 10.1289/ehp.10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walf AA, Frye CA. Estradiol reduces anxiety- and depression-like behavior of aged female mice. Physiol Behav. 2010;99:169–174. doi: 10.1016/j.physbeh.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang VC, Sable HJK, Ju YH, Allred CD, Helferich WG, Korol DL, Schantz SL. Effects of chronic estradiol treatment on delayed spatial alternation and differential reinforcement of low rates of responding. Behav Neurosci. 2008;122:794–804. doi: 10.1037/a0012513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang VC, Neese SL, Korol DL, Schantz SL. Chronic estradiol replacement impairs performance on an operant delayed spatial alternation task in young, middle-aged, and old rats. Horm Behav. 2009;56:382–390. doi: 10.1016/j.yhbeh.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weissenborn R, Robbins TW, Everitt BJ. Effects of medial prefrontal or anterior cingulated lesions on responding for cocaine under fixed-ratio and second-order schedules of reinforcement in rats. Psychopharmacology. 1997;134:242–257. doi: 10.1007/s002130050447. [DOI] [PubMed] [Google Scholar]
- 79.Wide JK, Hanratty K, Ting J, Galea LAM. High level estradiol impairs and low level estradiol facilitates non-spatial working memory. Behav Brain Res. 2004;51:45–53. doi: 10.1016/j.bbr.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 80.Winocur G, Hasher L. Aging and time-of-day effects on cognition in rats. Behav Neurosci. 1999;113:991–997. doi: 10.1037//0735-7044.113.5.991. [DOI] [PubMed] [Google Scholar]
- 81.Winocur G, Hasher L. Age and time-of-day effects on learning in a non-matching-to-sample test. Neurbiol Aging. 2004;25:1107–1115. doi: 10.1016/j.neurobiolaging.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 82.Wu JM, Zelinski MB, Ingram DK, Ottinger MA. Ovarian aging and menopause: current theories, hypotheses, and research models. Exp Biol Med. 2005;230:818–828. doi: 10.1177/153537020523001106. [DOI] [PubMed] [Google Scholar]
- 83.Young B, McNaughton N. Common firing patterns of hippocampal cells in a differential reinforcement of low rates of response schedule. J Neurosci. 2000;20:7043–7051. doi: 10.1523/JNEUROSCI.20-18-07043.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zurkovsky L, Boyd S, Brown SL, Fell JA, Korol DL. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience. 2007;144:26–37. doi: 10.1016/j.neuroscience.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


