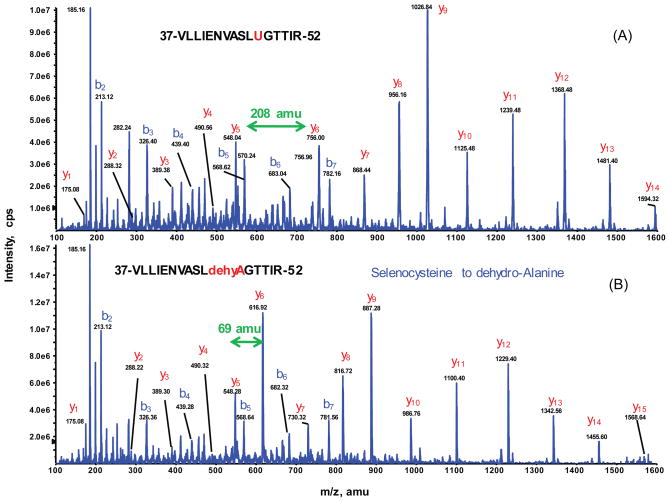
Fig. 2.
Identification of seleno-peptide for GPX1 by nanoLC-MS/MS. Representative MS/MS spectra of the doubly charged peptides ions at m/z [903.97]2+ for VLLIENVASLUGTTIR (A) and [834.46]2+ for VLLIENVASLdehydroAGTTIR (B), acquired from nanoLC-MS/MS analysis of tryptic digest of SOD1−/− gel band. All y-ion series including y6 and higher match to the GPX1 tryptic peptide covering residues #37–52 confirm that the residue 47 is Sec (selenocycteine) which is carbamidomethylated (208 amu) as shown in the top panel. The bottom panel shows that the same peptide sequence is unambiguously identified with the peptide mass decrease by 139amu. The MS/MS spectrum clearly indicates that the shift of −139amu begins at y6 and higher y-ion series, suggesting Sec at residue 47 be converted to DHA (dehydroalanine) (69 amu).