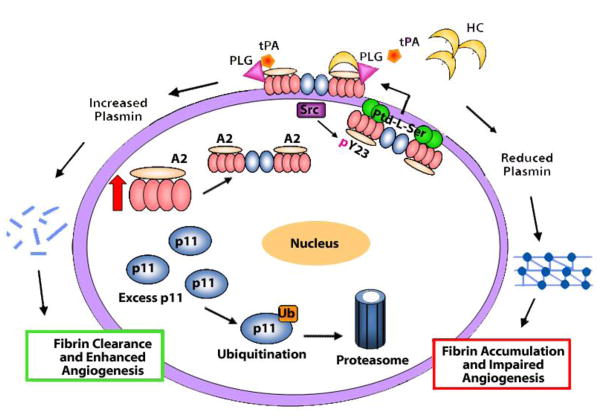
Figure 8. Model for A2 system dynamics in the endothelial cell.
When sufficient A2 is expressed, p11 binds to it, generating the A2 heterotetramer. In the absence of adequate A2, excess p11 is ubiquitinated and targeted for proteasomal degradation. Upon assembly of A2 and p11, the A2 heterotetramer is directed to the inner leaflet of the plasma membrane, where it may be phosphorylated at residue Y23 by activated pp60-c-src kinase (Src). Following phosphorylation, the A2 heterotetramer couples more tightly to inner leaflet anionic phosphatidylserine (Ptd-L-Ser), which may enable further conformational changes promoting translocation to the cell surface. Upon translocation, the A2 heterotetramer may serve as a co-receptor for tPA and plasminogen (Plg), stimulating plasmin production and fibrin clearance. However, in the presence of high levels of homocysteine (HC), A2 is derivatized by HC, which prevents tPA binding. As result, A2 cannot mediate plasmin generation at the endothelial cell surface, resulting in fibrin accumulation and impaired angiogenesis.