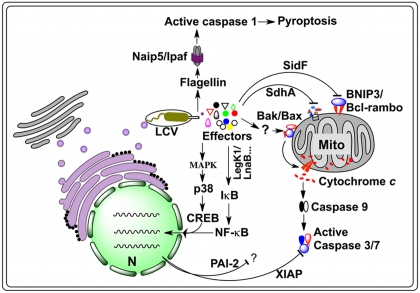
Figure 1.
Host cell death pathways targeted by L. Pneumophila. Internalized L. pneumophila translocates a large number of effectors into host cytosol via the Dot/Icm type IV secretion system. A yet unidentified set of effectors trigger an imbalance between the pro-death and pro-survival members of the Bcl-2 protein family, leading to the insertion of Bax/Bak into the mitochondrial out membrane, thus the release of cytochrome c and subsequent activation of the caspases 3 and 7. Another set of effectors, including LegK1 and LnaB, activate NF- B, most likely by initiating the kinase cascade that ultimately causes phosphorylation and subsequent degradation of I B, the inhibitor of NF- B, leading to nucleus translocation of NF- B and the induction of anti-apoptotic genes such as Xiap and Pai-2, whose product inhibits cell death by targeting caspases 3 and 7. Activation of the MAP kinase pathway by another set of unknown effectors can lead to similar effects. A third set of proteins such as SidF and SdhA, which inhibit host cell death by targeting pro-apoptotic proteins BNIP3 and Bcl-rambo or by unknown mechanisms. In non-permissive mouse macrophages, flagellin reached the cytosol probably via the Dot/Icm transporter is sensed by the NLR receptor Naip5, which together with Ipaf and the inflammasome activates caspase-1, leading to pyroptotic cell death. CREB, cAMP response element-binding protein; LCV, Legionella containing vacuole; MAPK, mitogen-activated protein kinases; Mito, mitochondrion; N, nucleus; Naip5, NLR family, apoptosis inhibitory protein 5; PAI-2, plasminogen activator inhibitor-2; XIAP, X-linked inhibitor of apoptosis protein.