Abstract
The cysteinyl transferase mycothiol ligase, or MshC, catalyzes the fourth step in the biosynthesis of the small molecular weight thiol mycothiol. MshC is essential for growth of Mycobacterium tuberculosis. Two groups of known aminoacyl tRNA synthetase inhibitors were evaluated for inhibition of M. tuberculosis MshC including aminoacyl adenosine analogs and natural products. Using enzyme assays, isothermal titration calorimetry and NMR, we show that MshC is selectively inhibited by cysteinyl sulfamoyl adenosine, and that discrimination occurs at the amino acid moiety.
Keywords: Mycothiol, Mycobacteria, STD NMR, Antituberculars, Aminoacyl adenosine
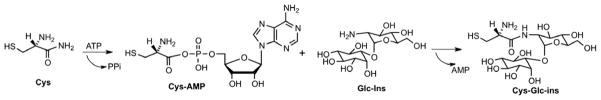
Mycothiol ligase, also referred to as MshC, is an enzyme essential for growth of Mycobacterium tuberculosis1,2 (Mtb) making it an attractive target for tuberculosis drug development.1,3,4 MshC catalyzes the ATP-dependent condensation of cysteine and glucosamine- α(1-1)myo-D-inositol (GlcN-Ins) to form cysteinyl GlcN-Ins (Cys-GlcN-Ins) (Fig. 1), an intermediate in the biosynthesis of mycothiol (AcCys-GlcN-Ins). In mycobacteria, mycothiol functions as a redox buffer and as a reserve of cysteine. Mycothiol is also involved in the detoxification of antibiotics and reactive oxygen and nitrogen species.3
Figure 1.
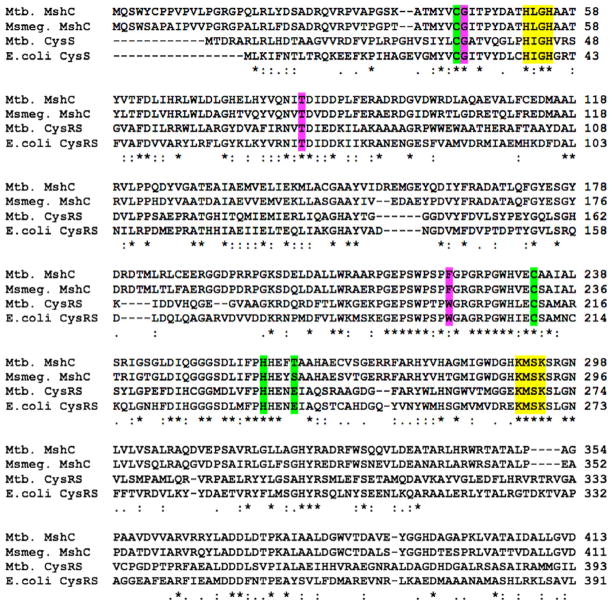
Alignment of Mtb and M. smegmatis MshC and Mtb and E. coli CysRS. Residues involved in ATP, Zn2+ and Cys binding are colored yellow, green and pink, respectively.
MshC was first purified from Mycobacterium smegmatis and shown to be the product of cysS2,5 a gene originally annotated as a second cysteinyl-tRNA synthetase (CysRS) in the Mtb genome.6 MshC from Mtb shares ~35% sequence homology with CysRS from Mtb and Escherichia coli (Fig. 2). Based on sequence homology and similarity of the reactions these enzymes catalyze, a two-step catalytic mechanism was proposed for MshC.5 The first step, common to CysRS and MshC, includes adenylation of cysteine by ATP to form the activated cysteinyl-AMP (Cys-AMP) intermediate. For CysRS, the second step involves transfer of cysteine to the ribose unit at the 3′ terminus of tRNAcys to produce the charged cysteinyl ester.7 By comparison the second step for MshC involves amide bond formation between the activated carbonyl carbon of Cys-AMP and the free amino group of GlcN-Ins to form Cys-GlcN-Ins (Fig. 2).5 In addition, kinetic and mechanistic characterization of M. smegmatis MshC revealed that MshC follows a ‘bi uni uni bi ping-pong’ steady-state kinetic mechanism where ATP and then Cys are bound by MshC to generate the cysteinyl-AMP intermediate and pyrophosphates, and release of pyrophosphates occurs prior to binding and transfer of GlcN-Ins to Cys-AMP.8
Figure 2.
Reaction catalyzed and product formed by mycothiol ligase.
The crystal structure of M. smegmatis MshC in complex with the bi-substrate analog 5′-O-[N-(L-cysteinyl) sulfamoyl] adenosine (1, CysSA) was recently reported.9 In addition to revealing MshC to be structurally homologous to CysRS with the catalytic domain possessing a Rossmann fold, the structure showed that the thiol group of 1 interacts with a zinc ion at the base of the active site suggesting that it participates in amino acid binding and discrimination as shown previously for E. coli CysRS.10 Despite its utility as an antitubercular target, little is known about small molecule inhibitors of MshC. E. coli CysRS is known to be highly selective for activation of Cys, discriminating against its serine isostere by more than 20,000-fold at the binding step due in part to the higher affinity of the zinc–thiolate interaction over the zinc–hydroxyl interaction.10,11
To investigate susceptibility of MshC to known aminoacyl tRNA (aa-tRNA) synthetase inhibitors, we evaluated the effects of two types of inhibitors toward MshC. These included the three aminoacyl adenosine analogs CysSA (1), SerSA (2) and AspSA (3), and four natural product inhibitors ochratoxin A (4), borrelidin (5), cispentacin (6), and mupirocin (7) (Fig. 3). Compounds 1–3 are mechanistic inhibitors of the first catalytic step of CysRS, SerRS, and AspRS, respectively,12 and the natural product inhibitors mimic to varying extents substrates and/or intermediates of other aminoacyl adenosines. Though unrelated structurally, mupirocin and isoleucyl sulfamoyl adenosine (IleSA) bind IleRS similarly with the epoxy, tetrahydropyran and C-13 hydroxyl of mupirocin occupying the same regions as the phosphate, ribose and isoleucine moieties of Ile-AMP, respectively.12,13 Borrelidin inhibits E. coli ThrRS at the step of threonine activation in a non-competitive manner with respect to threonine and ATP, yet was suggested to bind at the back of the active site to disrupt zinc ion coordination and ThrRS activity.14 Ochratoxin A contains a phenylalanine unit and inhibits Thermus thermophilus PheRS.12 Interestingly, when the phenylalanine of ochratoxin is replaced by valine, the resulting compound inhibits ValRS.12 The proline analog cispentacin [(1R,2S)-2-aminocyclopentane-1-carboxylic acid] weakly inhibits Candida albicans ProRS, and a closely related analog icofungipen [(1R,2S)-2-amino-4-methylidene-cyclopentane] inhibits C. albicans IleRS.15
Figure 3.
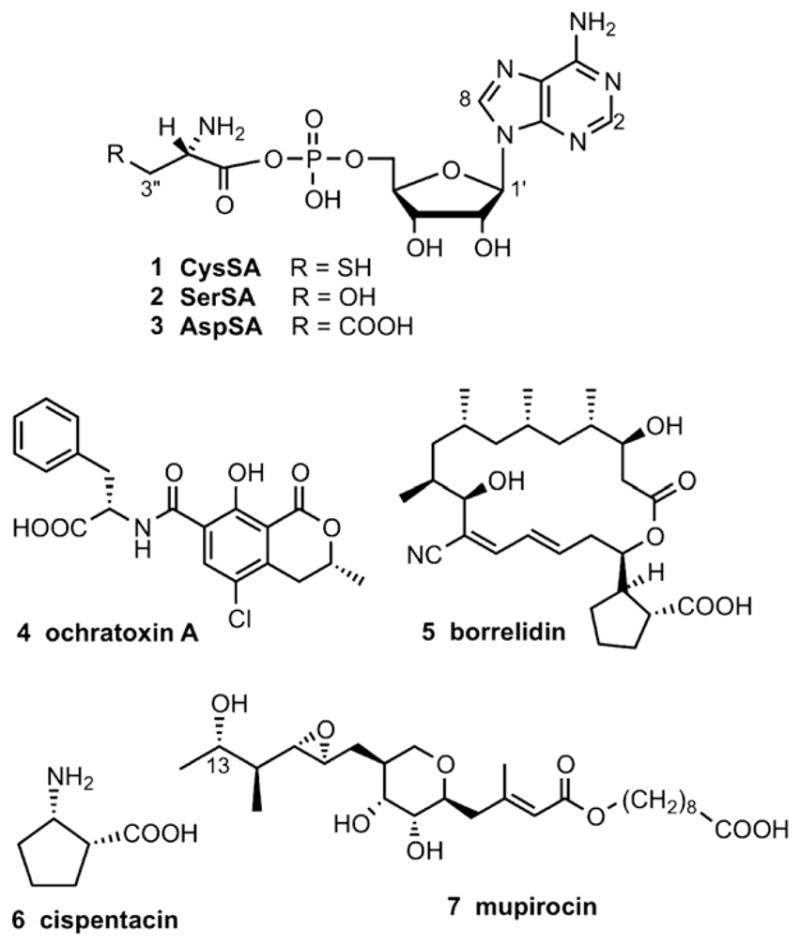
Known tRNA synthetase inhibitors.
Inhibitors were tested at concentrations ranging from nM to mM in a fluorescence detected HPLC assay that measures formation of fluorescently labeled Cys-GlcN-Ins5 using recombinant maltose binding protein (MBP)-MshC produced as described previously.16 The results are shown in Table 1. Of the known aa-tRNA synthetase inhibitors, only CysSA had an inhibitory effect on the activity of MshC at reasonable concentrations, showing an IC50 value of 50 nM. These data suggest that MshC shows comparable discrimination for thiol-containing inhibitors, similar to CysRS. The remaining six compounds showed no effect until doses reached mM concentrations.
Table 1.
Inhibition of MshC
| Compound | IC50 | Aminoacyl-t-RNA synthethase |
|---|---|---|
| 1 CysSA | 50 nM | CysRS |
| 2 SerSA | 5 mM | AspRS |
| 3 AspSA | 1.2 mM | SerRS |
| 4 Ochratoxin A | 2.5 mM | PheRS |
| 5 Borrelidin | >2 mM | ThrRS |
| 6 Cispentacin | >4 mM | ProRS |
| 7 Mupirocin | >5 mM | IleRS |
Data from inhibition assays employing compounds 2 and 3 nevertheless gave standard dose–response curves showing no signs of aggregation or precipitation at mM concentrations. Thus we next used isothermal titration calorimetry (ITC) to measure equilibrium association constants, and Saturation Transfer Difference (STD)17 NMR and NOESY or transferred NOESY (trNOESY) experiments to compare the mode of binding of CysSA versus AspSA. First, ITC measurements showed CysSA to bind MshC with a KD of 48.5 nM, comparable to the observed 50 nM IC50 value. trNOESY spectra of CysSA free in solution and in the presence of MBP-MshC further confirmed binding as cross peaks changed in sign from positive to negative, respectively (Supplementary, Figs. S1-2). In contrast NOESY spectra of AspSA in the presence of MBP-MshC did not contain any cross peaks (SI, Fig. S3) suggesting absence of binding. No heats of binding were detected with ITC, a method that is poor at detecting weak binding (mM Kds), consistent with weak inhibition.
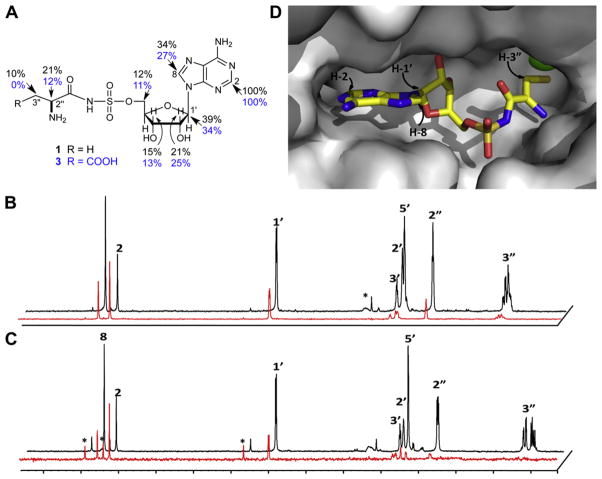
Next, separate 1H STD NMR spectra of 1 and 3 were recorded in the presence of MBP-MshC and the soluble MBP tag as control to determine the epitopes used for binding to M. tuberculosis MshC.18,19 STD NMR is an especially powerful technique because it can be used to assign binding epitopes of ligands that bind their macromolecular receptors over a large spectrum of affinities ranging from high mM to sub-nM.17 The 1H STD spectra of CysSA and AspSA in the presence of MBP-MshC are shown in Figure 4. Comparison of signals in the two STD spectra with those of the corresponding reference spectrum showed adenine H-2 (δ 8.15) to be most strongly enhanced for both analogs. (No binding was observed for either CysSA or AspSA to the soluble MBP tag.) Thus integrated peaks in the difference spectra were normalized to H-2 and the relative enhancements for CysSA and AspSA compared. As seen in Figure 4A, comparable enhancements for the purine and ribose moieties of 1 and 3 were observed with H-2 and H-1′ showing the strongest enhancements. In contrast, differences in enhancements within the amino acid moiety are apparent. Relative enhancements for H-2″ of CysSA (δ 3.91) and AspSA (δ 3.80) are 21% and 12%, respectively. The H-3″ (δ 2.94) proton of 1 showed a 10% relative enhancement while no effect could be observed for H-3″ (δ 2.65) of 3.
Figure 4.
Binding of CysSA and AspSA to MshC by STD NMR. (A) Chemical structure of CysSA and AspSA with relative enhancements (%) shown in black and blue, respectively. STD NMR spectra19,20 of (B) CysSA and (C) AspSA in the presence of MshC with reference and difference spectra colored black and red, respectively. Artefacts due to water suppression or impurities are marked with asterisks. Spectra were recorded as described in detail in Ref. 19. Weaker binding of AspSA to MshC relative to CysSA is apparent from the respective signal-to-noise (S/N) ratios of 20 and 90 in the difference spectra. S/N ratios for the CysSA and AspSA reference spectra are 170. Spectra were normalized to H-2. (D) Close up of CysSA in complex with M. smegmatis MshC with hydrogens in closest contact with the protein labeled.
These data are consistent with interactions observed in the crystal structure of M. smegmatis MshC9 in complex with CysSA where the purine H-2 is directed deep in the binding pocket, followed by H-8 and H-1′ that are positioned further from the surface of the protein (Fig. 4D). In addition, these results indicate that although the adenosine portion of CysSA and AspSA, and presumably other adenosyl-containing compounds, can bind MshC through the adenosyl portion of the binding site, the discrimination observed for this enzyme occurs at the amino acid unit. Thus, although the GlcN-Ins acceptor used by MshC differs significantly in structure from the tRNA substrates of tRNA synthetases, the Cys-AMP binding domains exhibit comparable selectivity. A comparison of aminoacyl tRNA synthetases co-crystallized with aminoacyl adenosyl analogs showed that the adenosine and ribose moieties adopt similar orientations in the active site of most class I and II aa-tRNAs,20 with H-2 and H-1′ in close proximity to the protein, consistent with our STD data. In terms of MshC inhibitor design, our results suggest utility for adenosine analogs and/or small molecule thiolates, perhaps in the form of prodrugs, as MshC inhibitors.
Supplementary Material
Acknowledgments
This work was supported by the NIH Intramural Research Program (NIDDK) and the Intramural AIDS Targeted Antiviral Program, Office of the Director, NIH (C.A.B.).
Footnotes
Supplementary data (NOESY spectra) associated with this article can be found, in the online version, at doi:10.1016/j.bmcl.2011.02.042.
References and notes
- 1.Sareen D, Newton GL, Fahey RC, Buchmeier NA. J Bacteriol. 2003;185:6736. doi: 10.1128/JB.185.22.6736-6740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sassetti CM, Boyd DH, Rubin EJ. Mol Microbiol. 2003;48:77. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- 3.Newton J, Buchmeier N, Fahey R. Microbiol Mol Biol Rev. 2008;72:471. doi: 10.1128/MMBR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gutierrez-Lugo MT, Baker H, Shiloach J, Boshoff H, Bewley CA. J Biomol Screen. 2009;14:643. doi: 10.1177/1087057109335743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sareen D, Steffek M, Newton GL, Fahey RC. Biochemistry. 2002;41:6885. doi: 10.1021/bi012212u. [DOI] [PubMed] [Google Scholar]
- 6.Cole ST, et al. Nature. 1998;393:537. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 7.Ibba M, Söll D. Annu Rev Biochem. 2000;69:617. doi: 10.1146/annurev.biochem.69.1.617. [DOI] [PubMed] [Google Scholar]
- 8.Fan F, Luxenberger A, Painter GF, Blanchard JS. Biochemistry. 2007;46:11421. doi: 10.1021/bi7011492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tremblay LW, Fan F, Vetting MW, Blanchard JS. Biochemistry. 2008;47:13326. doi: 10.1021/bi801708f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang CM, Christian T, Newberry KJ, Perona JJ, Hou YM. J Mol Biol. 2003;327:911. doi: 10.1016/s0022-2836(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 11.Rulíšek L, Havlas Z. J Am Chem Soc. 2000;122:10428. [Google Scholar]
- 12.Hurdle JG, O’Neill AJ, Chopra I. Antimicrob Agents Chemother. 2005;49:4821. doi: 10.1128/AAC.49.12.4821-4833.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakama T, Nureki O, Yokoyama S. J Biol Chem. 2001;276:47387. doi: 10.1074/jbc.M109089200. [DOI] [PubMed] [Google Scholar]
- 14.Ruan B, Bovee ML, Sacher M, Stathopoulos C, Poralla K, Francklyn CS, Söll D. J Biol Chem. 2005;280:571. doi: 10.1074/jbc.M411039200. [DOI] [PubMed] [Google Scholar]
- 15.Tao J, Schimmel P. Expert Opin Invest Drugs. 2000;9:1767. doi: 10.1517/13543784.9.8.1767. [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez-Lugo MT, Newton GL, Fahey RC, Bewley CA. Protein Expr Purif. 2006;50:128. doi: 10.1016/j.pep.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Meyer B, Peters T. Angew Chem, Int Ed. 2003;42:864. doi: 10.1002/anie.200390233. [DOI] [PubMed] [Google Scholar]
- 18.Lam SN, Acharya P, Wyatt R, Kwong PD, Bewley CA. Bioorg Med Chem. 2008;16:10113. doi: 10.1016/j.bmc.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NMR samples were prepared in Shigemi tubes containing 300 μL of 20 μM MBP-MshC (or MBP tag) and 900 μM CysSA or AspSA in NMR buffer (20 mM NaCl, 20 mM phosphate, pH 6.5 in 99.9% D2O). The NMR buffer for CysSA also contained 2 mM dithiothreitol-d10. Detailed descriptions of the experimental NMR parameters used can be found in Ref. 18.
- 20.Protein Data Bank identifiers for representative aminoacyl tRNA synthetases in complex with cognate aminoacyl adenosyl analogs include 2V0C (LeuRS/LeuSA), 2CT8 (MetRS/MetSA), 1N78 (GluRS/GluSA), 1JZQ (IleRS/IleSA), 1IVS (ValRS/ValSA), 2ZUE (ArgRS/ArgSA), 3CMQ (PheRS/PheSA) and 1FYF (ThrRS/SerSA).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.