1. Introduction
Although the majority of applications of gene therapy for human diseases have relied upon direct administration into the target tissue, systemic administration is generally thought to be more reliable, easy, and more appealing, particularly for diseases that affect multiple tissues. This is especially true for cancers. However, when one considers systemic routes with gene therapy vectors, multiple challenges exist, related to tropism/targeting and effects of the circulating humoral and cellular host defenses against such vectors. One exciting avenue that has been exploited by multiple groups recently has employed mammalian cells as a carrier for such gene therapy vectors [1-3]. Such carrier cells possess the advantage of hiding the vector from circulating humoral and cellular defense mediators, and, in some cases, have been shown to be targetable to the tissue of interest, particularly in the case of tumors [4-6]. In this chapter, we plan to review the current state of the art in cell-mediated delivery of such vectors to tumors, especially focusing on adenovirus and herpes simplex virus type 1 (HSV-1), where mesenchymal and neural stem cells have been shown to be engineered to act as carriers.
2. Carrier cell types
The innate and adaptive immune system can be an efficient host defense, largely responsible for eliminating circulating naked virions before they reach a tumor. It is widely accepted that a more efficient delivery system for naked virions is needed to improve their therapeutic efficacy, especially against metastatic or diffusely infiltrating tumors. Attempts to use cells to deliver anti-cancer agents date back nearly two decades [7]. Autologous host mammalian cells would not be recognized as foreign by host immunity, and thus hiding an oncolytic virus (OV) within them could provide a solution to the elimination of systemically delivered OVs. Ideally, the carrier cells should be able to target or home to the tumor. Interestingly, mounting evidence shows that stem and progenitor cells, immune cells, and cancer cells themselves possess such tumor-homing characteristics [3,6,8,9]. While this homing by immune cells and cancer cells does not seem surprising, more remarkable has been the discovery that multipotent tissue cells, such as mesenchymal and neural stem cells, are attracted to microenvironments that possess abnormal vascular structures, necrotic hypoxia and/or inflammation, possibly through the sensing of chemoattractive molecules (Fig. 1) [10]. Since this environmental milieu is often found in malignant tumors, stem cell-based delivery of genes and viruses is becoming a widely used strategy for experimental cancer therapy. In the next sections, we will discuss the different types of stem cells employed for such strategies.
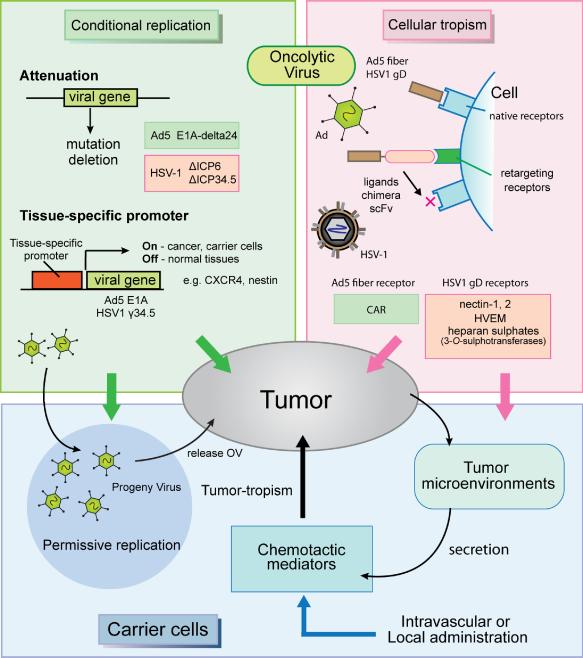
Fig. 1.
Schematic overviews of the carrier cell-based oncolytic virus (OV) delivery to tumors. Cellular tropism (purple shaded region): Cellular internalization of Adenovirus serotype 5 (Ad5) and herpes simplex viruses type 1 (HSV-1) depends on cellular receptor expressions: Coxsackie and Adenovirus Receptor (CAR) for Ad5 fiber and nectin-1, 2, HVEM and several 3-O-sulphotransferase-modified heparan sulphates for HSV-1 gD . Cellular retargeting is achieved by viral ligand modifications by means of covalent linkage to peptides that can target cellular receptors, by using chimeras with other adenoviral serotype proteins or by using single-chain antibodies (scFv) to link adenoviral entry to a specific cellular receptor. Conditional replication (green shaded region): Ad5 E1A mutation and transcriptional regulation by tissue-specific promoter (e.g. CXCR4) achieve conditional viral replication. Tumor-specific HSV-1 replication is based on the deletions of UL39 gene (ICP6) and/or γ34.5 genes (ICP34.5), and conditional γ34.5 gene transcription by tumor-specific promoters (e.g. nestin). Carrier cells (blue shaded region): mesenchymal/neural stem cells (MSC, NSC), immune cells (T cells), or cancer cells themselves have been used as carrier cells to deliver Ovs, usually through intravascular (intravenous and carotid artery) or local (e.g. intracranial) administration. Carrier cells can migrate to tumor beds attracted by chemotactic mediators secreted from the tumor microenvironment. Carrier cells allow OV replication to release progeny OVs toward tumors.
a. Mesenchymal Stem Cells (MSCs)
Mesenchymal stem cells (MSCs) are non-hematopoietic adult multipotent stem cells that can be isolated from many sources including the bone marrow (BM), adipose tissue, peripheral blood (PB), umbilical cord blood (UCB), bone, muscle and cartilage, and expanded with relative ease on plastic tissue culture dishes [11]. In the presence of specific induction factors, MSCs differentiate into mesodermal cells such as adipocytes, chondrocytes and osteoblasts. Their hypo-immunogenic status combined with their ability to regenerate damaged tissue has been exploited through transplantation of allogeneic MSCs to treat several degenerative diseases [12].
Interest in MSCs for cell-mediated cancer gene therapy was spawned by showing that they can home to tumors. Studeny M et al. demonstrated tumor tropism of human MSCs in a metastatic melanoma to murine lungs model, where interferon (INF)-β gene-loaded hMSCs generated an inhibitory effect on tumor growth and improvement of animal survival [3]. In addition to intravenous administration, adoptively transferred MSCs, derived from the bone marrow, adipose or umbilical cord tissue, have also been shown to possess a natural propensity for migration into engrafted tumors in mice [10]. This homing ability of MSC toward tumors is thought to be due to chemotactic mediators (cytokines and chemokines, etc.) that are also secreted by wounded tissues. Suggestive evidence for this chemotaxis comes from the finding that MSCs express receptors for these mediators on their surface [13].
Paradoxically, in the sites of increased angiogenesis most solid tumors are also characterized by poor vascular perfusion and hypoxia. Interestingly, these regions of hypoxia in tumors are also thought to harbor cells resistant to both chemotherapy and radiotherapy [14]. Low oxygen conditions activate hypoxia-inducible factors (HIF)-mediated gene transcription that can further increase the secretion of proangiogenic and inflammatory cytokines involved in MSC recruitment [15]. In addition, hypoxia is also known to increase MSC proliferation and diminish their differentiation capacity. It also has been found that co-injection of MSCs with tumor cells supported MSC proliferation in s.c. melanoma xenograft [16]. Collectively, these findings suggest that exogenously delivered MSC could home to solid tumors and, if MSCs carry a transgene or virus, these findings also imply that such therapeutic transgenes or viruses would also target the tumor microenvironments. In fact, the feasibility of this delivery approach for OVs has been validated by several investigators, utilizing oncolytic adenovirus and myxoma virus in metastatic breast carcinomas, ovarian cancer and malignant glioma in mice (Table 1) [4,5,17-20].
Table 1.
Mesenchymal/Neural stem carrier cells based OV delivery
| Virus name | Promoter/gene | Fiber | Administration | Cancers | Reference |
|---|---|---|---|---|---|
| MSC | |||||
| Ad5/3 | wt/E1A | 5/3 | Intraperitoneal | Metastasis ovarian cancer | Komzrova S [19] |
| Ad5/3.CXCR4 | CXCR4/E1A | 5/3 | Tail vein | Lung-metastasis breast cancer | Stoff-Khaliki MA [17] |
| Intracranial | glioma in brain | Sonabend AM [18] | |||
| Ad5.pk7-Δ24 | wt/E1A-delta24 | pK7 | Intravenous | Lung-metastasis breast cancer | Hakkarainen T [4] |
| Ad5-Δ24RGD | RGD | ||||
| Δ24-RGD |
wt/E1A-delta24 |
RGD |
Internal carotid artery |
glioma in brain |
Yong RL [5] |
| NSC | |||||
| hrR3 (HSV-1) | ΔUL39 | - | Intratumoral | glioma in brain | herrlinger U [2] |
| Ad-S-pk7 | Survivin/E1A | pK7 | Intracranial | glioma in brain | Tyler MA [38] |
Although the aforementioned studies have reported success in the capacity of homing MSC to tumors and also in delivery of MSCs loaded with viral and anticancer agents to neoplasms, this capacity has also been called into question by others. In fact, intravenous delivery of MSCs has been reported to home to a broad range of organs without evidence of specificity toward tumors in mice, rat or human [21]. Hakkarainen et al. have reported that intravenously injected OV-loaded MSCs homed rapidly to tumor-bearing lungs followed by delayed virus accumulation to the animal hepatic system [4]. Notwithstanding this lack of specificity, they were still able to detect a significant survival advantage by intravenous administration of OV-loaded MSCs in tumor-bearing mice when compared with naked OVs or replication-deficient Ad. They suggested that the OV-loaded MSCs might be releasing OVs from circulation into tumors even if they were not directly homing to tumors. In a brain tumor model, MSCs were also reported to have a lack of tropism toward intracranially grafted tumors via tail vein injection [22]. However, to bypass this lack of specificity, Yong et al. showed that GFP-labeled MSCs loaded with a replication-deficient Ad could localize into intracranially grafted human glioma after administration through the carotid artery in mice, leading to a significant improvement in the survival of brain tumor-bearing mice after OV-loaded MSC administration [5]. Therefore, the lack of specificity of tumor homing might be circumvented by directly administering MSCs into the arterial system of the targeted organ. In fact, a decade ago, we and others were able to show that naked OV administration to brain tumors was more efficacious after direct carotid administration than after intravenous administration [23-25]. Obviously, one could circumvent concerns about non-specific homing of MSCs by direct injection into the target tumor or organ: in fact, stereotactic intracranial injection of MSCs has been reported to lead to migration of the MSCs even into xenografted gliomas located a distance away from the site of injection [18]. Another concern voiced about MSCs has been that they could possibly change the kinetics of tumor growth because of cytokine/growth factor release, potential provision of a supportive microenvironment for tumor cells and/or potential induction of neo-angiogenesis [21]. In summary, it seems that additional work is needed to characterize the issue of carrier cell homing and delivery of biologic products, as well as possible effects that the carrier cell may have on tumor biology.
b. Neural stem cells (NSC)
Neural stem cells (NSCs), by definition, are characterized by their ability to differentiate into cells of the nervous system (neurons, astrocytes or oligodendrocytes) and by their capacity to self-renew [26]. In the adult brain, the dentate gyrus of the hippocampus and the subventricular zone (SVZ) of the lateral ventricle define the locations for neural stem or progenitor cells, where neurons are born. A recent study also suggests that neural progenitor cells also lie in neocortical layers [27].
NSCs can not only migrate throughout the brain during development, but can also migrate toward acute lesions, such as stroke, and areas of neurodegenerative damage. Based on the findings by several groups that NSCs also displayed tumor tropism, therapeutic applications of NSCs have also focused on cancer [1,2,8]. It has been reported that tumor microenvironments, where hypoxia is common and where there can be promotion of neo-angiogenesis, release cytokines, growth factors and proangiogenic factors (such as SDF-1, FGF2, VGEF and HGF) that can enhance NSC mobilization [14,28]. In addition, endothelial cells, but not vascular smooth muscle cells, have been reported to directly enhance the self-renewal of NSCs in vitro [29]. Similar to what has been reported with MSCs, lower oxygen tension can promote NSC proliferation and suppress its differentiation. Thus, the tropism toward the hypoxic regions of neoplasms may likely result from NSCs’ attempts in response to ischemic injury to be neuroprotective and to migrate towards such areas in order to induce neurogenesis and effectuate neural repair [30,31]. NSC can also down-regulate effector functions of inflammatory T cells and macrophages, in response to pro-inflammatory cytokines such as TNFα, IL-1β and IFNν, and can promote neuroprotection in CNS regions exposed to inflammation through expression of death receptor ligands (such as FasL, TRAIL and APO3L) that induce apoptosis of inflammatory T lymphocytes [33]. NSC transplantation studies also suggest that most implanted cells express markers of an undifferentiated state (such as nestin), thus suggesting that they may not actually be replacing injured local cells through a differentiation process but may rather be delaying neurodegeneration and suppressing inflammation through release of neural growth and immunomodulatory factors [34].
In addition to this reported capacity for migration towards tumor and/or areas of neural damage, NSCs have also been documented to actually suppress tumor growth. Glass et al. reported that endogenous neural precursor cells from the SVZ displayed tropism toward glioblastomas grafted in animal cortex [32]. Interestingly, they discovered that there was increased aggressiveness of glioblastomas with older rats that correlated with the decline of neural precursors and their ability to migrate towards the tumors, again suggestive of a tumor-suppressive effect mediated by neural precursors. Intravenously administered NSCs can also form perivascular niches, reside for up to three month in the mice CNS and move out toward regions of the brain that are affected by pathology [33]. Experiments have shown that brain tumor stem cells also reside within perivascular niches, thus implying that intravascular delivery of NSCs may target them. Therefore, intravascular administration of NSCs may also be an attractive route of delivery to target brain tumors [35]. Interestingly, tumor tropism of NSCs has also been reported for other cancer models (melanoma and breast carcinoma) [36,37]. A number of investigators have reported that genetically armed NSCs have delivered anticancer agents such as prodrug-activating enzymes and suicide genes to implanted tumors after intravenous or intracranial administration routes [28].
Few investigators have explored the use of NSCs to carry OVs to cancer targets (Table 1). Herrlinger et al. utilized herpes simplex virus type 1 (HSV-1) loaded into v-myc immortalized mouse neural precursors (NPCs). When these loaded NPCs were implanted into mouse brains they reported NPC migration towards xenografted gliomas [2]. More recently, Tyler et al. demonstrated that c-myc immortalized human NSCs loaded with a conditionally replicative adenovirus (CRAd) migrated towards the site of implanted tumors and improved the survival of tumor-bearing mice [38].
The notion of an eventual clinical use for NSCs is challenged by the limited source of primary cells. NSCs, immortalized by introducing oncogenes such as v-myc and c-myc, remain a safety concern because of potential degeneration into tumors. It may be that well-characterized allogeneic cell lines could be of sufficient safety to be considered for future clinical use [28], and NSCs derived from cultured pluripotent stem cells (embryonic stem cells- ES-, and induced pluripotent stem cells-iPSC) may become the most readily accessible and accepted source for future clinical trials [39].
3. Improving the transduction of carrier cells
The process of loading carrier cells with OVs or gene therapy vectors has received relatively limited attention. Efficiency in this process would greatly improve the development of large preclinical and clinical batches of such cells for trials in humans. In this section, we will focus on the areas where such efficiency has been investigated.
a. Cellular tropism
Oncolytic viruses need to be internalized into carrier cells and thus require the presence of surface binding receptors. Adenovirus serotype5 (Ad5) is the most widely used conditionally replication-competent adenovirus (CRAd). It requires the binding of the cellular coxsackie-adenovirus receptor (CAR) to its knob portion of the fiber protein [40]. The attachment of the Ad fiber protein to CAR on the cell surface is an important first step in infection. Although naked Ad5-based gene delivery and oncolysis has been widely used, most potential cell carriers and malignant tumors express low levels of CAR [18,41]. Chemical and genetic modifications of adenovirus have been researched to overcome Ad5's limited entry into carrier cells. In the following paragraphs, such viral modifications will be addressed and reviewed (Fig. 1).
a) PEGylated adenovirus
Chemical conjugation with polyethylene glycol (PEG), a hydrophilic and nonimmunogenic polymer, was initially developed in order to shield virions from neutralizing antibodies and also permits dose reduction to limit virus-induced host inflammatory responses. Because PEGylation of adenovirus also interfered with CAR binding, there was reduced infection of off-target hepatic cells, upon systemic delivery in vivo [41]. Recently, attaching peptides and ligands to the ends of PEG chains has yielded tissue-specific gene transfer. The Arg-Gly-Asp (RGD) peptide motif binds specifically to αv integrins, which are frequently over-expressed on the cell surface of tumor and tumor-associated endothelium. This approach has also been exploited to modify PEGylated Ad to achieve targeting of integrins ανβ3 on endothelial and tumor cells [41]. In addition to small peptides such as RGD, PEG conjugation to larger ligands such as epidermal growth factor (EGF) has been demonstrated to enhance Ad gene transfer to EGFR-positive cells [42]. In this study, EGF protein conjugated to biotin-PEG, complexed with avidin on the surface of Ad, and enhanced gene transduction in EGF Receptor (EGFR)-expressing epithelial carcinoma cells. This gene transfer was suppressed by the presence of free EGF or, in EGFR-negative cells, indicating the specificity of EGFR targeting. Such peptides conjugated to PEGylated Ads might potentially be exploited to deliver various viruses to different carrier cells based on their receptor expression.
b) Polycation mediated internalization to increase viral transduction
Viral gene transfer efficiency is influenced by electrostatic repulsion between the negatively charged cell surface and the net negatively charged virion. Hence, neutralization of membrane charges to bridge virion and cell surface should augment gene transduction. Polybrene, one of the polycation agents, is used to improve retrovirus and lentivirus-mediated gene transductions in vitro. This strategy has also been used in adenovirus-mediated gene transfer. The use of a polycation-based transfection reagent has also been shown to significantly improve viral entry in cultured MSC without affecting their viability [43,44]. This simple method can achieve efficient gene transduction and viral progeny without having to undergo complex genetic modifications.
c) Fiber-modified Adenoviruses
Although chemical modification to increase viral transduction efficiency is promising, the expansion to large-scale production is cumbersome and may make quality control at each step of conjugation and production of subsequent reagent batches a challenging process for clinical use. Therefore, virus retargeting strategies by genetic modification of the Ad capsid have been investigated extensively.
Early studies showed that the external peptides at the carboxy terminus of the Ad fiber protein can change their tropism against targeting cells, while a HI loop exposed to the outside of the fiber knob domain can flexibly incorporate the target ligands without disturbing the intramolecular interactions of the knob during trimerization process, which is required for Ad entry [45]. These results suggested possible retargeting constructions of the oncolytic Ad. Integrin ανβ3 retargeting of Ad5-delta24RGD is one such tropism-modified CRAd which is currently being evaluated in human patients for safety and efficacy (http://www.clinicaltrials.gov/ct2/show/NCT00562003). Ad-RGD can improve gene transduction in MSCs that express negligible CAR. Recently, intravenous administration of human MSC loaded with Ad-RGD was found to be able to efficiently deliver and release virus particles to intracerebral orthotopic glioma and lead to improved survival of the tumor-bearing mice [5,46].
Similarly, a stretch of seven lysine residues [K7 (KKKKKKK) peptide] that possess heparan sulfate targeting motif have been incorporated in the HI-loop of fiber protein of adenovirus (Ad-p7k). This Ad-p7k also achieved high gene transduction and increased therapeutic efficacy in several cancer models in mice [47-49]. This fiber-modified virus also displayed a more than 400-fold increase of gene transduction in MSC compared to Ad5, and a 16-fold increased gene transduction relative to Ad-RGD [17]. Neural stem cells are known to express modest levels of CAR on their cell surface: Ad-p7k was able to significantly improve gene transduction in these cells [38]. In addition, malignant glioma cells decreased CAR expression level, and treatment with CRAd-p7k enhanced therapeutic efficacy [49]. Collectively, these studies indicate the potential for utilizing fiber-modified viruses to increase transduction efficacy of carrier cells.
d) Chimeric fiber protein with other serotypes
More than 100 different Ads have been isolated and categorized into six subgroups (A to F). Among these, subgroup B Ads (e.g. Ad3, 7, 11 and 35) can utilize CD46 that is ubiquitously expressed on cell surfaces, and other as yet unidentified proteins as receptors for entry into host cells. To increase transduction efficiency of subgroup C Ad5 vectors, genetic engineering has been utilized to replace subgroup C knob domain or shaft/knob domains with that of subgroup B Ad fibers, in order to achieve infection in a broad range of cell types. In addition to improving internalization, chimeric capsids can also reduce liver tropism, reducing the risk of hepatotoxicity, and may also increase circulation time when compared to unmodified Ad5 [50,51]. Thus, capsid-modified Ad vectors overcome the limitation of natural Ad5 in entry into carrier cells.
Ad5 vectors containing chimeric Ad5 tail and Ad3 shaft/knob (Ad5/3) or Ad35 shaft/knob (Ad5F35) have been thus loaded into carrier cells to target tumors. For ovarian and breast cancers, oncolytic Ad5/3 enhanced the lytic effects more than Ad-RGD and Ad5. Intravenous delivery of Ad5F35-loaded MSCs improved the survival of mice from their metastatic cancers [17,19].
Surprisingly, MSCs, which express relatively low levels of CD46, were also found to be permissive to Ad5F35 infection, suggesting that other unidentified receptors were involved in Ad5F35 entry [52]. Apart from these carrier cells, Ad5F35 also exhibited advanced gene transduction and oncolysis for malignant gliomas, when compared to Ad5-RGD and Ad5/3, bolstering the potential for using chimeric viruses for carrier cell-mediated tumor therapy [53].
e) Cellular retargeting for HSV-1
Unlike adenoviruses, HSV-1 has not been extensively studied for cellular retargeting. Only recently have there been studies investigating HSV1 retargeting. HSV-1 entry is initiated first by its attachment to the host cell through viral envelope proteins glycoprotein B (gB) or glycoprotein C (gC) to heparan-sulphate chains on cell-surface heparin sulfate proteoglycans. This is subsequently followed by more specific binding of gD and gB to specific cell-surface receptors, such as immunoglobulin-like type 2 receptor-α (PILRα) (for gB) and nectin-1, -2, HVEM and heparan sulphate produced by several 3-O-sulphotransferases (for gD) resulting in viral envelope and cell membrane fusion [54,55]. HSV-1 gD has been modified to create retargeted recombinant viruses. Zhou and Roizman genetically engineered the viral gD receptor to create a chimeric gD with IL-13 or urokinase plasminogen activator (uPA) proteins to retarget HSV-1 infection to IL13 or uPA-expressing tumor cells [56]. More recently Menotti et al. created HER2 (human epidermal growth factor receptor 2)-retargeted HSV-1 vectors by conjugation of a single-chain antibody (scFv) to HER2 [57]. This scFv approach would be an attractive candidate to rapidly expand the engineering of retargeted viruses for cancer gene therapy.
b. Conditional replication in tumor and carrier cells
After entry into cells, OVs will initiate the process of replication that leads to progeny virion production and release with ensuing death of the infected cell. This process could be relatively short (12-18 hours for HSV1) or long (24-48 hours for adenovirus or vaccinia virus). In addition, the carrier cell should be able to unload progeny viruses at the desired tumor site. In the ensuing paragraphs, we will briefly review how replication could affect the efficiency of OV loading into carrier cells and unloading into tumors (Fig. 1).
a) Tumor-selective adenovirus
Natural viruses, such as adenoviruses, drive quiescent normal cells to enter the cell cycle in order to replicate. Upon Ad infection, the E1 region gene products bind to Rb or p53 protein to disrupt their G1-S phase regulation. To de-target normal cells, oncolytic Ads have been genetically engineered to restrict viral replication based on the functionality of these pathways [116]. In fact, Ad-Δ24 and dl922-947 viruses with mutated pRB-binding region of E1A have been investigated to target cancers [58,59]. In addition, some papers reported the use of Ad-Δ24 in MSC-mediated delivery with subsequent Ad-Δ24 release into tumors [4,5,17].
To increase the tumor-specific potency of oncolytic viruses, tissue-specific promoters have been exploited to drive viral gene expressions. While several exciting strategies using tissue or cancer-specific promoters have been used to drive viral replication, detailed descriptions of all these promoters are beyond the scope of this article and are reviewed elsewhere [60,61]. Briefly, using bispecific promoters in carrier cells and targeting cancers, Hamada et al. employed an ovarian cancer cell-derived carrier cell loaded with an IAI.3B promoter driven CRAd and demonstrated cancer cell-mediated OV delivery with a therapeutic effect in ovarian tumors established in the flanks of Ad immunized mice [62]. In an analogous approach using normal carrier cells, the C-X-C chemokine receptor 4 (CXCR4) promoter-driven Ad was tested to be delivered by MSC to target breast and malignant glioma tumors in mice. Compared with wild-type E1A promoter, CXCR4 promoter-driven Ad replication was many times higher in both hMSCs and human glioma cell lines [18]. In a murine model of metastatic breast carcinoma, hMSC-mediated Ad-CXCR4 intravenous delivery increased survival rate compared to naked Ad injection [17]. Therefore, several approaches exist to ensure OV replication and release from carrier cells to tumor sites.
b) Oncolytic HSV-1 vectors
Attenuation of HSV-1 to de-target normal cells was initially achieved by mutants with defects in nucleic acid metabolism functions. One of the earliest developed mutants (hrR3 is an HSV-1 with a lacZ insertional mutation in the ICP6 locus of the UL39 gene product) lacked the large subunit of HSV-1 ribonuleotide reductase (RR), whose activity is required for efficient viral replication in normal non-dividing cells (but not in tumor cells [93]), and more recently was shown to replicate selectively in cells with p16 tumor suppressor gene defects regardless of cell cycle status [63]. Because NPCs divide and self-renew, they have been shown to be relatively permissive in allowing hrR3 to replicate [2]. An additional common deletion in HSV-1 has been to remove ICP34.5 (the γ2 34.5 gene product), responsible both for binding cellular protein phosphatase 1α (PP1α) that allows for protein synthesis, and beclin-1 that allows for cellular autophagy. This mutated HSV-1 has also been shown to lead to additional attenuation and prevention of neurovirulence [64,65]. Thus, a double-mutant HSV-1 virus (in both ICP6 and ICP34.5) could be delivered into a xenograft epithelial ovarian cancer (EOC) in mice by using irradiated teratocarcinoma cells as carriers [9].
While tumor-specific oncolytic HSV-1 viruses have been created, the efficacy of loading them up in carrier cells utilizing chemical modification with PEG has not yet been investigated [66,67]. In addition, future development of bispecific promoter-driven viruses, able to target both the carrier cells and the cancer cells, may improve the release of progeny OVs and therapeutic efficacy.
4. Extending the life span of virus-loaded carrier cells
Although oncolytic viruses are attenuated by a variety of stratagems to restrict replication in normal tissues, most cells utilized as carriers are relatively permissive for viral replication, partly due to the fact that they self-renew and cycle (MSCs and NSCs). Therefore, the survival time of a virus-loaded carrier cell is limited [2,18,20]. It is difficult to reliably regulate viral replication in the carrier cells so that timely release of the OV load occurs in the tumor, rather than at other sites trafficked by the loaded carrier before, during or after administration. Intravenous administrations of virus-loaded carrier cells reach the neovasculature in tumors, yet have been reported to not deliver the OV load as efficiently as hoped [4]. The poor prognosis of patients with malignant tumors such as glioblastoma multiforme, advanced pancreatic adenocarcinoma and diffuse-type gastric carcinoma is also due to tumor cells located relatively far from vessels, further impeding the effect of an OV released by carrier cells in blood vessels [68,69]. This problem is also illustrated by the anticancer failures of conventional drug delivery. In addition, abnormal blood flow by contorted and abnormally leaky tumor vessels causes heterogeneous distribution of carrier cells [14]. Therefore, the half-life of a loaded carrier cell may be a very critical factor for the success of this type of therapy.
To avoid cell lysis during delivery, Qiao et al researched whether membrane attachment of vesicular stomatitis virus (VSV) to carrier T cells, instead of cellular internalization, with low doses of VSV led to lack of replication in the carrier cell and allowed gradual release of the oncolytic VSV [6]. In fact, the surface-attached VSV seemed to reduce carrier cell lysis, but there was still some shielding of the adhered viruses from host immune responses during intravascular delivery in preimmune mice. Willmon et al. recently reviewed this method in detail [70].
Another published approach has been to employ an inhibitor of DNA synthesis to suppress virus replication after carrier cell loading [2]. Herrlinger et al. used mimosine to arrest the replication of the mutant HSV-1, rRp450, in loaded NPC carrier cells temporarily. This allowed time for neural precursor cell (NPC) migration to tumors in the brain. With mimosine, they reported that the loaded rRp450 virus was retained in the carrier NPCs for more than 2 weeks with restriction of viral replication until mimosine was removed. These NPCs were still able to migrate to the intracerebral glioma after intratumoral injection in mouse brains.
While these methods appear to work relatively well in the experimental systems, additional permutations may be even more effective. It is possible, in fact, that additional genetic engineering of loaded OVs could be attempted in order to achieve a virus whose replication would be restricted in the carrier cell until it reached its tumor target.
5. Conclusion
Carrier cell-based delivery of OVs and genes requires the integration of multiple types of expertise, from viral design to carrier cell preparation, from kinetics of cell and viral life cycles to pharmacokinetics. Future efforts in improving all of these research areas may lead to efficient clinical applications of this technology.
Acknowledgments
Financial Support:
This work was supported by funding from the National Institutes of Health Grant K01NS059575; R01NS064607; R21NS056203 to BK, and R21NS0632901; U01 NS061811; P01 CA069246 to EAC.
Biography
 E. Antonio Chiocca, MD, PhD has been the chair of the Ohio State University Medical Center Department of Neurological Surgery since 2004. He holds the Dardinger Family Endowed Chair in Oncological Neurosurgery at the James Comprehensive Cancer Center. He was previously an associate professor of Neurosurgery at Massachusetts General Hospital/Harvard Medical School (MGH/Harvard), where he also completed his residency. He obtained his MD/PhD from the University of Texas Medical School in Houston in 1988. He has published more than 220 papers and chapters related to glioma biology and treatment. He has held continuous NIH funding since 1995 and has also been funded by numerous private foundations for his research on gliomas. He was awarded the Grass Foundation Award by the Society for Neurological Surgery in 2007 and the Farber Award for Brain Tumor Research by the Society for Neurooncology in 2008. He was elected to the American Society for Clinical Investigation (ASCI) in 2005 and as a fellow of the American Association for the Advancement of Sciences (AAAS) in 2005. He has been a member of the National Cancer Institute (NCI) – D (clinical studies) parent committee and of NCI's Developmental Therapeutics Study Section. He also sits on the scientific advisory board for the American Brain Tumor Association, the Goldhirsh Foundation and the Sonntag foundation. He sits on the editorial board of several journals, including Molecular Therapy, Journal of Neurosurgery and Neurosurgery. His research has focused on developing novel treatments for brain tumors, including gene-based and virus-based therapies. He has also worked on mechanistic aspects of brain tumor biology.
E. Antonio Chiocca, MD, PhD has been the chair of the Ohio State University Medical Center Department of Neurological Surgery since 2004. He holds the Dardinger Family Endowed Chair in Oncological Neurosurgery at the James Comprehensive Cancer Center. He was previously an associate professor of Neurosurgery at Massachusetts General Hospital/Harvard Medical School (MGH/Harvard), where he also completed his residency. He obtained his MD/PhD from the University of Texas Medical School in Houston in 1988. He has published more than 220 papers and chapters related to glioma biology and treatment. He has held continuous NIH funding since 1995 and has also been funded by numerous private foundations for his research on gliomas. He was awarded the Grass Foundation Award by the Society for Neurological Surgery in 2007 and the Farber Award for Brain Tumor Research by the Society for Neurooncology in 2008. He was elected to the American Society for Clinical Investigation (ASCI) in 2005 and as a fellow of the American Association for the Advancement of Sciences (AAAS) in 2005. He has been a member of the National Cancer Institute (NCI) – D (clinical studies) parent committee and of NCI's Developmental Therapeutics Study Section. He also sits on the scientific advisory board for the American Brain Tumor Association, the Goldhirsh Foundation and the Sonntag foundation. He sits on the editorial board of several journals, including Molecular Therapy, Journal of Neurosurgery and Neurosurgery. His research has focused on developing novel treatments for brain tumors, including gene-based and virus-based therapies. He has also worked on mechanistic aspects of brain tumor biology.
 Dr. Balveen Kaur majored in physics at Delhi University and proceeded to obtain an M.S. in biotechnology at Banaras Hindu University. She subsequently carried out her Ph.D. at Emory University, followed by a postdoctoral fellowship in the laboratory of Dr. Erwin Van Meir at Emory University where she studied the role of angiogenesis in the context of glioma progression. Much of her work focused on the tumor microenvironment and the antiangiogenic and antitumorigenic properties of endogenous inhibitors. She joined the faculty of The Ohio State University in 2005 as an Assistant Professor, where her laboratory is currently studying the role of the tumor microenvironment and angiogenesis as limiting factors for glioma virotherapy.
Dr. Balveen Kaur majored in physics at Delhi University and proceeded to obtain an M.S. in biotechnology at Banaras Hindu University. She subsequently carried out her Ph.D. at Emory University, followed by a postdoctoral fellowship in the laboratory of Dr. Erwin Van Meir at Emory University where she studied the role of angiogenesis in the context of glioma progression. Much of her work focused on the tumor microenvironment and the antiangiogenic and antitumorigenic properties of endogenous inhibitors. She joined the faculty of The Ohio State University in 2005 as an Assistant Professor, where her laboratory is currently studying the role of the tumor microenvironment and angiogenesis as limiting factors for glioma virotherapy.
 Hiroshi Nakashima received his Ph.D degree at Osaka University Graduate School of Medicine in Japan in 2005. From 2001 to 2004 he was also a pre-doctoral research fellow in the Japan Society for the Promotion of Science and a visiting graduate student at Nagoya University in Japan, researching mammalian chromosome organization and chromatin structures using human artificial chromosome technology. In 2006, he started as a postdoctoral researcher in Dr. Chiocca's Dardinger laboratory, in the Department of Neurological Surgery at James Comprehensive Cancer Center and The Ohio State University Medical Center. He was a recipient of the 2007 travel award at the American Society of Gene Therapy. He has been working on the development of brain cancer gene therapy using stem cell carriers and oncolytic viruses.
Hiroshi Nakashima received his Ph.D degree at Osaka University Graduate School of Medicine in Japan in 2005. From 2001 to 2004 he was also a pre-doctoral research fellow in the Japan Society for the Promotion of Science and a visiting graduate student at Nagoya University in Japan, researching mammalian chromosome organization and chromatin structures using human artificial chromosome technology. In 2006, he started as a postdoctoral researcher in Dr. Chiocca's Dardinger laboratory, in the Department of Neurological Surgery at James Comprehensive Cancer Center and The Ohio State University Medical Center. He was a recipient of the 2007 travel award at the American Society of Gene Therapy. He has been working on the development of brain cancer gene therapy using stem cell carriers and oncolytic viruses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, Breakefield XO, Snyder EY. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herrlinger U, Woiciechowski C, Sena Esteves M, Aboody KS, Jacobs AH, Rainov NG, Snyder EY, Breakefield XO. Neural precursor cells for delivery of replication-conditional HSV-1 vectors to intracerebral gliomas. Mol Ther. 2000;1:347–357. doi: 10.1006/mthe.2000.0046. [DOI] [PubMed] [Google Scholar]
- 3.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 4.Hakkarainen T, Sarkioja M, Lehenkari P, Miettinen S, Ylikomi T, Suuronen R, Desmond RA, Kanerva A, Hemminki A. Human mesenchymal stem cells lack tumor tropism but enhance the antitumor activity of oncolytic adenoviruses in orthotopic lung and breast tumors. Hum Gene Ther. 2007;18:627–641. doi: 10.1089/hum.2007.034. [DOI] [PubMed] [Google Scholar]
- 5.Yong RL, Shinojima N, Fueyo J, Gumin J, Vecil GG, Marini FC, Bogler O, Andreeff M, Lang FF. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas. Cancer Res. 2009;69:8932–8940. doi: 10.1158/0008-5472.CAN-08-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiao J, Kottke T, Willmon C, Galivo F, Wongthida P, Diaz RM, Thompson J, Ryno P, Barber GN, Chester J, Selby P, Harrington K, Melcher A, Vile RG. Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy. Nat Med. 2008;14:37–44. doi: 10.1038/nm1681. [DOI] [PubMed] [Google Scholar]
- 7.Culver KW, Ram Z, Wallbridge S, Ishii H, Oldfield EH, Blaese RM. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992;256:1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- 8.Benedetti S, Pirola B, Pollo B, Magrassi L, Bruzzone MG, Rigamonti D, Galli R, Selleri S, Di Meco F, De Fraja C, Vescovi A, Cattaneo E, Finocchiaro G. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–450. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- 9.Coukos G, Makrigiannakis A, Kang EH, Caparelli D, Benjamin I, Kaiser LR, Rubin SC, Albelda SM, Molnar-Kimber KL. Use of carrier cells to deliver a replication-selective herpes simplex virus-1 mutant for the intraperitoneal therapy of epithelial ovarian cancer. Clin Cancer Res. 1999;5:1523–1537. [PubMed] [Google Scholar]
- 10.Lee DH, Ahn Y, Kim SU, Wang KC, Cho BK, Phi JH, Park IH, Black PM, Carroll RS, Lee J, Kim SK. Targeting rat brainstem glioma using human neural stem cells and human mesenchymal stem cells. Clin Cancer Res. 2009;15:4925–4934. doi: 10.1158/1078-0432.CCR-08-3076. [DOI] [PubMed] [Google Scholar]
- 11.Oreffo RO, Cooper C, Mason C, Clements M. Mesenchymal stem cells: lineage, plasticity, and skeletal therapeutic potential. Stem Cell Rev. 2005;1:169–178. doi: 10.1385/SCR:1:2:169. [DOI] [PubMed] [Google Scholar]
- 12.Abdallah BM, Kassem M. The use of mesenchymal (skeletal) stem cells for treatment of degenerative diseases: current status and future perspectives. J Cell Physiol. 2009;218:9–12. doi: 10.1002/jcp.21572. [DOI] [PubMed] [Google Scholar]
- 13.Spaeth E, Klopp A, Dembinski J, Andreeff M, Marini F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008;15:730–738. doi: 10.1038/gt.2008.39. [DOI] [PubMed] [Google Scholar]
- 14.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 15.Fehrer C, Brunauer R, Laschober G, Unterluggauer H, Reitinger S, Kloss F, Gully C, GaBner R, Lepperdinger G. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6:745–757. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- 16.Djouad F, Bony C, Apparailly F, Louis-Plence P, Jorgensen C, Noel D. Earlier onset of syngeneic tumors in the presence of mesenchymal stem cells. Transplantation. 2006;82:1060–1066. doi: 10.1097/01.tp.0000236098.13804.0b. [DOI] [PubMed] [Google Scholar]
- 17.Stoff-Khalili MA, Rivera AA, Mathis JM, Banerjee NS, Moon AS, Hess A, Rocconi RP, Numnum TM, Everts M, Chow LT, Douglas JT, Siegal GP, Zhu ZB, Bender HG, Dall P, Stoff A, Pereboeva L, Curiel DT. Mesenchymal stem cells as a vehicle for targeted delivery of CRAds to lung metastases of breast carcinoma. Breast Cancer Res Treat. 2007;105:157–167. doi: 10.1007/s10549-006-9449-8. [DOI] [PubMed] [Google Scholar]
- 18.Sonabend AM, Ulasov IV, Tyler MA, Rivera AA, Mathis JM, Lesniak MS. Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma. Stem Cells. 2008;26:831–841. doi: 10.1634/stemcells.2007-0758. [DOI] [PubMed] [Google Scholar]
- 19.Komarova S, Kawakami Y, Stoff-Khalili MA, Curiel DT, Pereboeva L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol Cancer Ther. 2006;5:755–766. doi: 10.1158/1535-7163.MCT-05-0334. [DOI] [PubMed] [Google Scholar]
- 20.Josiah DT, Zhu D, Dreher F, Olson J, McFadden G, Caldas H. Adipose-derived Stem Cells as Therapeutic Delivery Vehicles of an Oncolytic Virus for Glioblastoma. Mol Ther. 2009 Nov;:10. doi: 10.1038/mt.2009.265. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lazennec G, Jorgensen C. Concise review: adult multipotent stromal cells and cancer: risk or benefit? Stem Cells. 2008;26:1387–1394. doi: 10.1634/stemcells.2007-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bexell D, Gunnarsson S, Tormin A, Darabi A, Gisselsson D, Roybon L, Scheding S, Bengzon J. Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. Mol Ther. 2009;17:183–190. doi: 10.1038/mt.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rainov NG, Zimmer C, Chase M, Kramm CM, Chiocca EA, Weissleder R, Breakefield XO. Selective uptake of viral and monocrystalline particles delivered intra-arterially to experimental brain neoplasms. Hum Gene Ther. 1995;6:1543–1552. doi: 10.1089/hum.1995.6.12-1543. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda K, Ichikawa T, Wakimoto H, Silver JS, Deisboeck TS, Finkelstein D, Harsh GR, 4th, Louis DN, Bartus RT, Hochberg FH, Chiocca EA. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5:881–887. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 25.Schellingerhout D, Rainov NG, Breakefield XO, Weissleder R. Quantitation of HSV mass distribution in a rodent brain tumor model. Gene Ther. 2000;7:1648–1655. doi: 10.1038/sj.gt.3301272. [DOI] [PubMed] [Google Scholar]
- 26.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 27.Ohira K, Furuta T, Hioki H, Nakamura KC, Kuramoto E, Tanaka Y, Funatsu N, Shimizu K, Oishi T, Hayashi M, Miyakawa T, Kaneko T, Nakamura S. Ischemia-induced neurogenesis of neocortical layer 1 progenitor cells. Nat Neurosci. 2009 Dec;:27. doi: 10.1038/nn.2473. 2009. [DOI] [PubMed] [Google Scholar]
- 28.Aboody KS, Najbauer J, Danks MK. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008;15:739–752. doi: 10.1038/gt.2008.41. [DOI] [PubMed] [Google Scholar]
- 29.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 30.Zhao D, Najbauer J, Garcia E, Metz MZ, Gutova M, Glackin CA, Kim SU, Aboody KS. Neural stem cell tropism to glioma: critical role of tumor hypoxia. Mol Cancer Res. 2008;6:1819–1829. doi: 10.1158/1541-7786.MCR-08-0146. [DOI] [PubMed] [Google Scholar]
- 31.Burns TC, Verfaillie CM, Low WC. Stem cells for ischemic brain injury: a critical review. J Comp Neurol. 2009;515:125–144. doi: 10.1002/cne.22038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glass R, Synowitz M, Kronenberg G, Walzlein JH, Markovic DS, Wang LP, Gast D, Kiwit J, Kempermann G, Kettenmann H. Glioblastoma-induced attraction of endogenous neural precursor cells is associated with improved survival. J Neurosci. 2005;25:2637–46. doi: 10.1523/JNEUROSCI.5118-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, Comi G, Constantin G, Martino G. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436:266–271. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- 34.Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7:395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- 35.Gilbertson RJ, Rich JN. Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 36.Brown AB, Yang W, Schmidt NO, Carroll R, Leishear KK, Rainov NG, Black PM, Breakefield XO, Aboody KS. Intravascular delivery of neural stem cell lines to target intracranial and extracranial tumors of neural and non-neural origin. Hum Gene Ther. 2003;14:1777–1785. doi: 10.1089/104303403322611782. [DOI] [PubMed] [Google Scholar]
- 37.Frank RT, Edmiston M, Kendall SE, Najbauer J, Cheung CW, Kassa T, Metz MZ, Kim SU, Glackin CA, Wu AM, Yazaki PJ, Aboody KS. Neural stem cells as a novel platform for tumor-specific delivery of therapeutic antibodies. PLoS One. 2009;4:e8314. doi: 10.1371/journal.pone.0008314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tyler MA, Ulasov IV, Sonabend AM, Nandi S, Han Y, Marler S, Roth J, Lesniak MS. Neural stem cells target intracranial glioma to deliver an oncolytic adenovirus in vivo. Gene Ther. 2009;16:262–278. doi: 10.1038/gt.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 40.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 41.Xiong Z, Cheng Z, Zhang X, Patel M, Wu JC, Gambhir SS, Chen X. Imaging chemically modified adenovirus for targeting tumors expressing integrin alphavbeta3 in living mice with mutant herpes simplex virus type 1 thymidine kinase PET reporter gene. J Nucl Med. 2006;47:130–139. [PMC free article] [PubMed] [Google Scholar]
- 42.Park JW, Mok H, Park TG. Epidermal growth factor (EGF) receptor targeted delivery of PEGylated adenovirus. Biochem Biophys Res Commun. 2008;366:769–774. doi: 10.1016/j.bbrc.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 43.Fouletier-Dilling CM, Bosch P, Davis AR, Shafer JA, Stice SL, Gugala Z, Gannon FH, Olmsted-Davis EA. Novel compound enables high-level adenovirus transduction in the absence of an adenovirus-specific receptor. Hum Gene Ther. 2005;16:1287–1297. doi: 10.1089/hum.2005.16.1287. [DOI] [PubMed] [Google Scholar]
- 44.Bosch P, Fouletier-Dilling C, Olmsted-Davis EA, Davis AR, Stice SL. Efficient adenoviral-mediated gene delivery into porcine mesenchymal stem cells. Mol Reprod Dev. 2006;73:1393–1403. doi: 10.1002/mrd.20593. [DOI] [PubMed] [Google Scholar]
- 45.Krasnykh V, Dmitriev I, Mikheeva G, Miller CR, Belousova N, Curiel DT. Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob. J Virol. 1998;72:1844–1852. doi: 10.1128/jvi.72.3.1844-1852.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lamfers M, Idema S, van Milligen F, Schouten T, van der Valk P, Vandertop P, Dirven C, Noske D. Homing properties of adipose-derived stem cells to intracerebral glioma and the effects of adenovirus infection. Cancer Lett. 2009;274:78–87. doi: 10.1016/j.canlet.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 47.Ranki T, Kanerva A, Ristimaki A, Hakkarainen T, Sarkioja M, Kangasniemi L, Raki M, Laakkonen P, Goodison S, Hemminki A. A heparan sulfate-targeted conditionally replicative adenovirus, Ad5.pk7-Delta24, for the treatment of advanced breast cancer. Gene Ther. 2007;14:58–67. doi: 10.1038/sj.gt.3302830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rein DT, Breidenbach M, Wu H, Han T, Haviv YS, Wang M, Kirby TO, Kawakami Y, Dall P, Alvarez RD, Curiel DT. Gene transfer to cervical cancer with fiber-modified adenoviruses. Int J Cancer. 2004;111:698–704. doi: 10.1002/ijc.20295. [DOI] [PubMed] [Google Scholar]
- 49.Zheng S, Ulasov IV, Han Y, Tyler MA, Zhu ZB, Lesniak MS. Fiber-knob modifications enhance adenoviral tropism and gene transfer in malignant glioma. J Gene Med. 2007;9:151–160. doi: 10.1002/jgm.1008. [DOI] [PubMed] [Google Scholar]
- 50.Breidenbach M, Rein DT, Wang M, Nettelbeck DM, Hemminki A, Ulasov I, Rivera AR, Everts M, Alvarez RD, Douglas JT, Curiel DT. Genetic replacement of the adenovirus shaft fiber reduces liver tropism in ovarian cancer gene therapy. Hum Gene Ther. 2004;15:509–518. doi: 10.1089/10430340460745829. [DOI] [PubMed] [Google Scholar]
- 51.Seshidhar Reddy P, Ganesh S, Limbach MP, Brann T, Pinkstaff A, Kaloss M, Kaleko M, Connelly S. Development of adenovirus serotype 35 as a gene transfer vector. Virology. 2003;311:384–393. doi: 10.1016/s0042-6822(03)00161-2. [DOI] [PubMed] [Google Scholar]
- 52.Knaan-Shanzer S, van de Watering MJ, van der Velde I, Goncalves MA, Valerio D, de Vries AA. Endowing human adenovirus serotype 5 vectors with fiber domains of species B greatly enhances gene transfer into human mesenchymal stem cells. Stem Cells. 2005;23:1598–1607. doi: 10.1634/stemcells.2005-0016. [DOI] [PubMed] [Google Scholar]
- 53.Hoffmann D, Meyer B, Wildner O. Improved glioblastoma treatment with Ad5/35 fiber chimeric conditionally replicating adenoviruses. J Gene Med. 2007;9:764–778. doi: 10.1002/jgm.1076. [DOI] [PubMed] [Google Scholar]
- 54.Satoh T, Arii J, Suenaga T, Wang J, Kogure A, Uehori J, Arase N, Shiratori I, Tanaka S, Kawaguchi Y, Spear PG, Lanier LL, Arase H. PILRalpha is a herpes simplex virus-1 entry coreceptor that associates with glycoprotein B. Cell. 2008;132:935–944. doi: 10.1016/j.cell.2008.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iankov ID, Blechacz B, Liu C, Schmeckpeper JD, Tarara JE, Federspiel MJ, Caplice N, Russell SJ. Infected cell carriers: a new strategy for systemic delivery of oncolytic measles viruses in cancer virotherapy. Mol Ther. 2007;15:114–122. doi: 10.1038/sj.mt.6300020. [DOI] [PubMed] [Google Scholar]
- 56.Zhou G, Roizman B. Separation of receptor-binding and profusogenic domains of glycoprotein D of herpes simplex virus 1 into distinct interacting proteins. Proc Natl Acad Sci U S A. 2007;104:4142–4146. doi: 10.1073/pnas.0611565104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Menotti L, Cerretani A, Hengel H, Campadelli-Fiume G. Construction of a fully retargeted herpes simplex virus 1 recombinant capable of entering cells solely via human epidermal growth factor receptor 2. J Virol. 2008;82:10153–10161. doi: 10.1128/JVI.01133-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fueyo J, Gomez Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, Shi YX, Levin VA, Yung WK, Kyritsis AP. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 59.Heise C, Hermiston T, Johnson L, Brooks G, Sampson-Johannes A, Williams A, Hawkins L, Kirn D. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat Med. 2000;6:1134–1139. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- 60.Ko D, Hawkins L, Yu DC. Development of transcriptionally regulated oncolytic adenoviruses. Oncogene. 2005;24:7763–7774. doi: 10.1038/sj.onc.1209048. [DOI] [PubMed] [Google Scholar]
- 61.Hardcastle J, Kurozumi K, Chiocca EA, Kaur B. Oncolytic viruses driven by tumor-specific promoters. Curr Cancer Drug Targets. 2007;7:181–189. doi: 10.2174/156800907780058880. [DOI] [PubMed] [Google Scholar]
- 62.Hamada K, Desaki J, Nakagawa K, Zhang T, Shirakawa T, Gotoh A, Tagawa M. Carrier cell-mediated delivery of a replication-competent adenovirus for cancer gene therapy. Mol Ther. 2007;15:1121–1128. doi: 10.1038/sj.mt.6300128. [DOI] [PubMed] [Google Scholar]
- 63.Aghi M, Visted T, Depinho RA, Chiocca EA. Oncolytic herpes virus with defective ICP6 specifically replicates in quiescent cells with homozygous genetic mutations in p16. Oncogene. 2008;27:4249–4254. doi: 10.1038/onc.2008.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 65.Kramm CM, Chase M, Herrlinger U, Jacobs A, Pechan PA, Rainov NG, Sena-Esteves M, Aghi M, Barnett FH, Chiocca EA, Breakefield XO. Therapeutic efficiency and safety of a second-generation replication-conditional HSV1 vector for brain tumor gene therapy. Hum Gene Ther. 1997;8:2057–2068. doi: 10.1089/hum.1997.8.17-2057. [DOI] [PubMed] [Google Scholar]
- 66.Kambara H, Okano H, Chiocca EA, Saeki Y. An oncolytic HSV-1 mutant expressing ICP34.5 under control of a nestin promoter increases survival of animals even when symptomatic from a brain tumor. Cancer Res. 2005;65:2832–2839. doi: 10.1158/0008-5472.CAN-04-3227. [DOI] [PubMed] [Google Scholar]
- 67.Kanai R, Tomita H, Hirose Y, Ohba S, Goldman S, Okano H, Kawase T, Yazaki T. Augmented therapeutic efficacy of an oncolytic herpes simplex virus type 1 mutant expressing ICP34.5 under the transcriptional control of musashi1 promoter in the treatment of malignant glioma. Hum Gene Ther. 2007;18:63–73. doi: 10.1089/hum.2006.107. [DOI] [PubMed] [Google Scholar]
- 68.Sterman DH, Treat J, Litzky LA, Amin KM, Coonrod L, Molnar-Kimber K, Recio A, Knox L, Wilson JM, Albelda SM, Kaiser LR. Adenovirus-mediated herpes simplex virus thymidine kinase/ganciclovir gene therapy in patients with localized malignancy: results of a phase I clinical trial in malignant mesothelioma. Hum Gene Ther. 1998;9:1083–1092. doi: 10.1089/hum.1998.9.7-1083. [DOI] [PubMed] [Google Scholar]
- 69.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer. 2008;8:425–437. doi: 10.1038/nrc2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Willmon C, Harrington K, Kottke T, Prestwich R, Melcher A, Vile R. Cell carriers for oncolytic viruses: Fed Ex for cancer therapy. Mol Ther. 2009;17:1667–1676. doi: 10.1038/mt.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]