Figure 5.
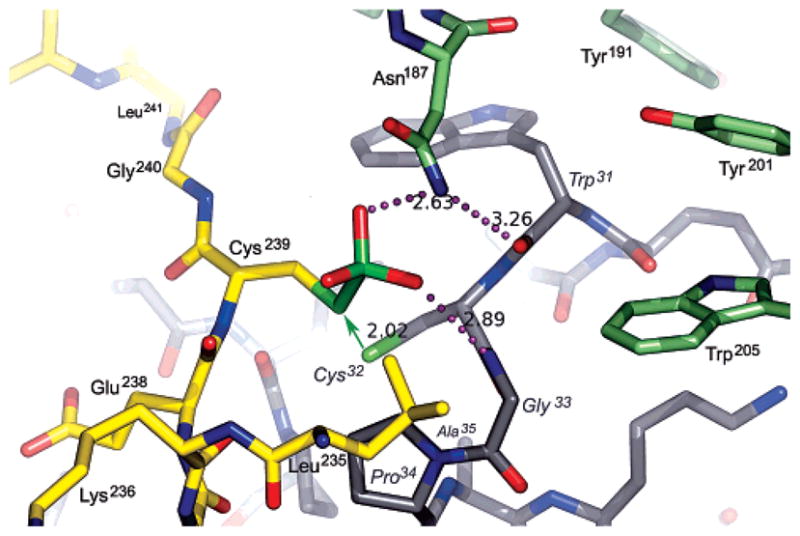
Model for the S-sulfocysteine intermediate of PAPS reductase bound to Trx1, based on the crystal structure of the mixed disulfide complex. The sulfite moiety fits into a pocket at the PAPS reductase–Trx1 interface. PAPS reductase residues in the C-terminal tail are colored yellow and other residues green; Trx1 residues are colored gray with labels in italics. Potential hydrogen bonds (dotted lines; distances in angstroms) include those from Asn187 to the sulfite and the carbonyl of Trp31 and from the amide of Gly33 to the sulfite. The green arrow represents the nucleophilic attack that must occur for formation of the mixed disulfide and displacement of sulfite; the distance between the Sγ atoms of Cys239 and Cys32 in the cocrystal structure is 2.02 Å. The resolving cysteine of Trx, Cys35, is mutated to Ala in the complex (visible below Pro34 in the 30s loop). With respect to Figure 3A, the view is approximately from the N-terminus of Trx1 looking along the axis of the C-terminal peptide.
