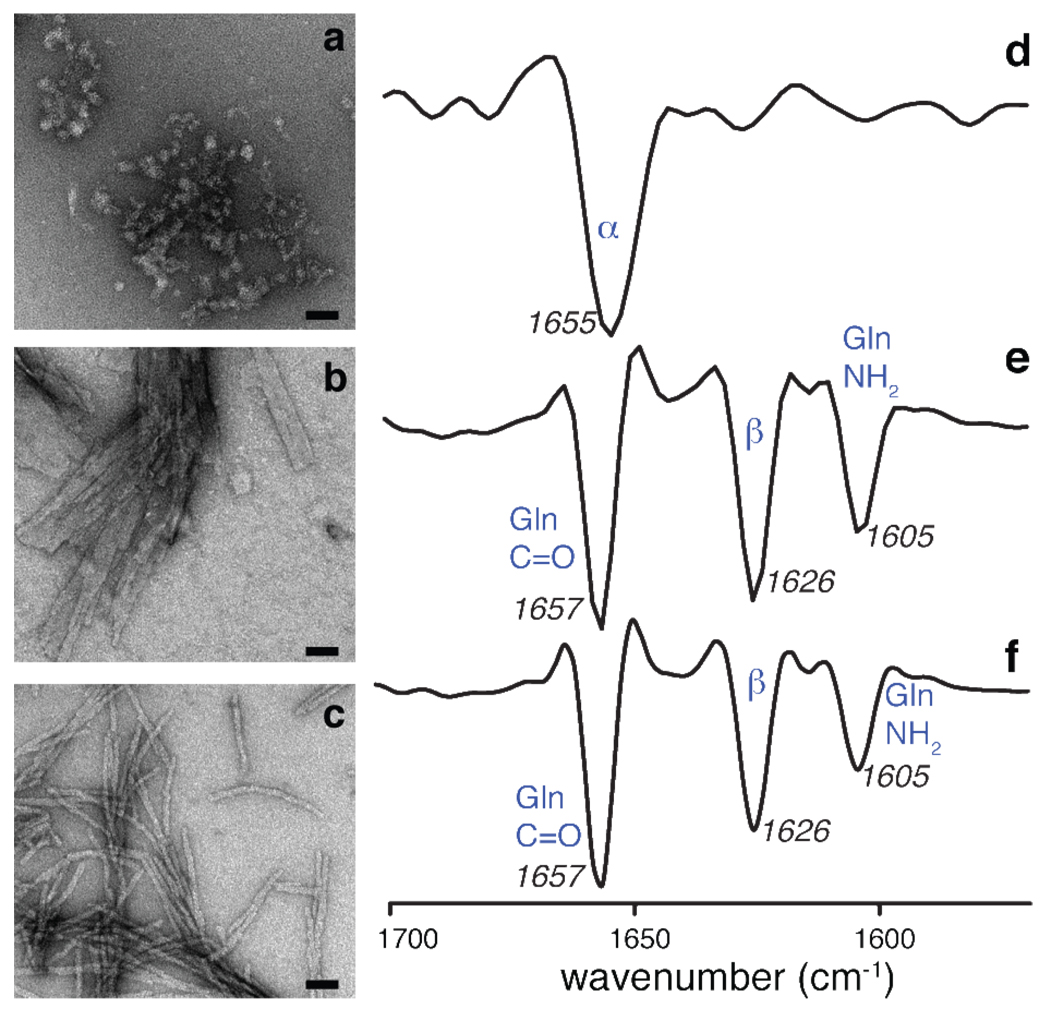
Figure 2.
Aggregate morphology and secondary structure. Negatively-stained transmission electron micrographs (left) and second-derivative FTIR data (right) of (a,d) httNT peptide aggregates, (b,e) mature fibrils formed by the polyQ model peptide K2Q31K2, and (c,f) httNTQ30P10K2. The TEM scale bars indicate a length of 50 nm. The httNT FTIR spectrum indicates substantial α-helical content, whereas that of the polyQ fibrils is dominated by the Gln signals and indicates β-sheet secondary structure10. Despite morphological differences by TEM, the httNTQ30P10K2 FTIR data strongly resemble the polyQ results. No helical signals can be unequivocally identified due to the overlap between the Gln signals and the helical band. Band assignments: (d) 1655 cm−1: α-helix; (e,f): 1657 cm−1: Gln side chain C=O stretch; 1626 cm−1: β-sheet structure; 1605 cm−1: Gln side chain NH2 deformation45,46.