Abstract
The Bacillus subtilis version of SCO1 (BsSCO) is required for assembly of CuA in cytochrome c oxidase and may function in thiol-disulfide exchange and/or copper delivery. BsSCO binds Cu(II) with ligation by two cysteines, one histidine and one water. However, copper is a catalyst of cysteine oxidation and BsSCO must avoid this reaction to remain functional. Time resolved, rapid freeze-quench (RFQ) electron paramagnetic resonance of apo-BsSCO reacting with Cu(II) reveals an initial Cu(II) species with two equatorially coordinated nitrogen atoms, but no sulfur. We propose that BsSCO evolves from this initial sulfur free coordination of Cu(II) to the final dithiolate species via a change in conformation, and that the initial binding by nitrogen is a means for BsSCO to avoid premature thiol oxidation.
Keywords: Copper binding, Rapid freeze quench EPR, SCO protein, SCO1 homolog from Bacillus subtilis
1. Introduction
Members of the SCO family of proteins have been implicated in the assembly of cytochrome c oxidase [1]. The SCO proteins have a conserved –CXXXC- motif and are proposed to be involved specifically in assembly of one of the copper centers (i.e., CuA) of the integral membrane enzyme cytochrome c oxidase [2]. The CuA center is composed of two copper ions held to the protein by a set of amino acids that includes a pair of bridging thiols from two cysteine residues. Assembly of CuA requires delivery of two copper ions to a site in which the side chains of the cysteine residues are in the reduced, dithiol, copper-binding state. SCO proteins, therefore, have been proposed to play a direct role in copper delivery and/or in disulfide exchange with the cysteine residues of the apo-CuA site [3,4]. There is evidence in vitro with the Thermus thermophilus version of SCO for a disulfide exchange role in formation of the CuA site in cytochrome ba3 oxidase. A second protein (i.e., PCuAC) is proposed to be the copper delivery vehicle in the Thermus system [5]. However, an equivalent of PCuAC is not found in Bacillus. It is still an open question as to whether the Bacillus subtilis version of SCO (i.e., BsSCO) functions in copper delivery and/or disulfide exchange. We have found that BsSCO binds Cu(II) extremely tightly, prefers Cu(II) over Cu(I), and proposed that the interaction with copper is a key aspect of BsSCO’s function [6].
Copper is well known as an oxidant of cysteine [7,8] and other thiol containing reagents [9,10]. Copper is also recognized as a reagent that promotes oxidative stress via its interaction with protein thiols [11]. In contrast the reaction of the reduced, dithiolate form of BsSCO with Cu(II) gives a stable complex with Cu(II) in quantitative yield [12]. The Cu(II) ion is ligated by two cysteines, one histidine and a water in the equilibrium complex [13]. We have shown in time-resolved studies using stopped-flow, UV–Vis absorption that the high affinity complex between BsSCO and Cu(II) is formed in a two-step process [14]. In the work reported here we characterized the intermediate state in the BsSCO/Cu(II) reaction by RFQ-electron paramagnetic resonance (EPR). We are able to observe development of the ligand binding site within BsSCO over time that supports the existence of two different protein conformers that provide two related, but distinct modes of copper binding. We observe the copper site on BsSCO evolve from an initial form with two nitrogen ligands and no sulfur to a site with two sulfurs and one nitrogen. We propose that the intermediate form serves to prevent premature thiol oxidation, and could be involved in metal exchange with the target (e.g., apo-CuA).
2. Materials and methods
2.1. Sample preparation
BsSCO was prepared and reduced as described in earlier work [6]. Reduced BsSCO (0.2 mM in 25 mM sodium phosphate buffer pH 7.0) was mixed (1:1 volume ratio) with equimolar aqueous CuCl2 and frozen after total effective incubation times of 10–140 ms at 23 ± 1 °C, using a modified and calibrated Update Instruments (Madison, WI) rapid-freeze-quench (RFQ) apparatus [15].
2.2. EPR spectroscopy
EPR was carried out at 9.6 and 34 GHz using a Bruker EleXsys E600 spectrometer equipped with Oxford Instruments flow-through helium cryostats. Precise frequencies were measured by inbuilt microwave counters. X-band EPR spectra were recorded at 10 mW, 62 K; other spectra (not shown) were recorded at 250 μW to 25 mW to determine the maximum non-saturating power. The Q-band spectrum presented was recorded at 50 μW, 74 K, and was indistinguishable from the derivative of the rapid-passage spectrum recorded with second-harmonic out-of-phase modulation at 60 mW, 63 K. Magnetic field modulation of 0.4 mT (4 G) at 100 kHz was employed. Computer simulations were carried out using XSophe [16]. Where structure was observed on the g⊥ line, A⊥ values for copper were refined starting with those suggested in the literature for square-planar based Cu(II) [17]. The approximation was made that copper couplings were due to 100% 63Cu. Quadrupolar terms were neglected because 63/65Cu quadrupolar terms are expected to be small compared to 14N superhyperfine lines and will affect only the weakest g⊥ features, due to secondary transitions, in the X- and Q-band spectra [18,19].
3. Results
3.1. Reaction of Cu(II) with BsSCO
In the absence of BsSCO, frozen aqueous CuCl2 exhibited an EPR signal (Fig. 1A and B) typical of Cu(II), with g|| = 2.406, g⊥ = 2.077 and A||Cu = 13.8 × 10−3 cm−1. These parameters indicate exclusively oxygen equatorial coordination (Fig. 2) [20]. Upon incubation of BsSCO with equimolar Cu(II) for 10–140 ms, similar and complex signals are observed that cannot be simulated assuming a single species (Fig. 1C, E and G). Upon further incubation of BsSCO with equimolar Cu(II), for 2 min and beyond, a characteristic signal is obtained that is best simulated, at both X- and Q-bands, by assuming a single equatorially coordinated nitrogen atom (Figs. 1I and J Fig. 3). Peisach and Blumberg analysis supports this assignment (Fig. 2). The lack of any obvious features due to the influence of a significant quadrupolar coupling suggest that there is no axially coordinated imidazole [19]. Slight differences in the values for the copper hyperfine interaction in the equatorial plane is observed in simulations at X- and Q-bands, and likely indicate some non-coincidence of g and A (Fig. 3).
Fig. 1.
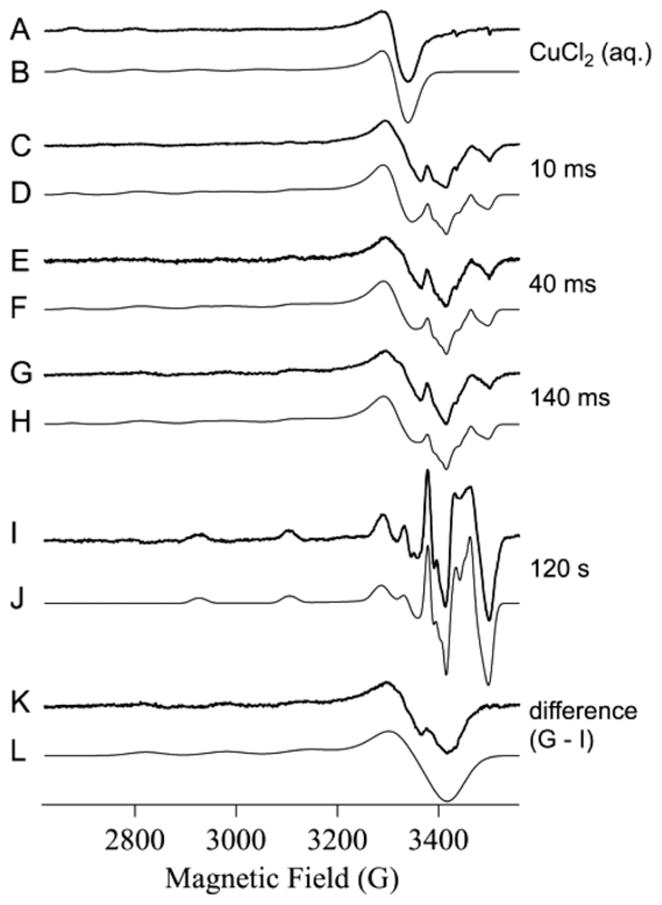
EPR spectra of Cu(II) and Cu(II) + BsSCO following incubation and rapid-freezing. Traces A, C, E, G, I and K are experimental X-band EPR spectra. Traces B, D, F, H, J, and L are computer simulations. Parameters used for the simulations were: B, g|| = 2.406, g⊥ = 2.077 and A|| Cu = 13.8 × 10−3 cm−1; J, g|| = 2.154, g⊥ = 2.033, Az,y,x Cu = 18.0, 4.5, 2.5 × 10−3 cm−1, and 1 × 14N with Az,y,x N = 1.1, 1.4, 1.1 × 10−3 cm−1; L, g|| = 2.251, g⊥ = 2.049, Az,y,x Cu = 16.6, 1.7, 2.6 × 10−3 cm−1, and 2 × 14N with Az,y,x N = 1.0, 1.4, 1.0 × 10−3 cm−1. For L, the values of Ax and Ay, for Cu and N, were not well determined; representative values were used and are given only for completeness. Traces D, F and H were computed by addition of B, J and L with fractional spin densities: D = (0.3 × B) + (0.1 × J) + (0.6 × L); F = (0.2 × B) + (0.1 × J) + (0.7 × L); H = (0.2 × B) + (0.1 × J) + (0.7 × L).
Fig. 2.
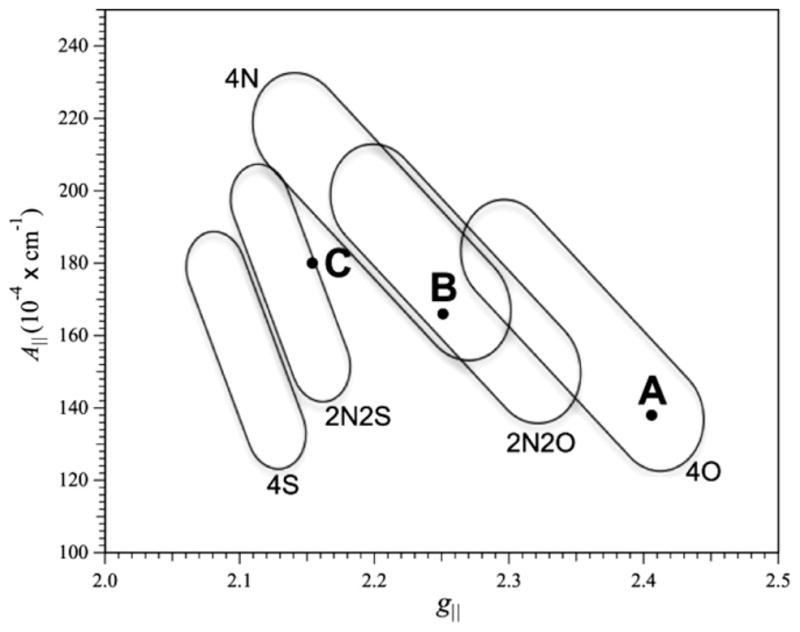
Peisach and Blumberg analysis of Cu(II) EPR spectra. The regions of the plot show the likely equatorial coordination of Cu(II) for species with ranges of values of g|| and A||. A corresponds to CuCl2, B to the intermediate species of Cu(II)–BsSCO, and C to the equilibrium species of Cu(II)–BsSCO.
Fig. 3.
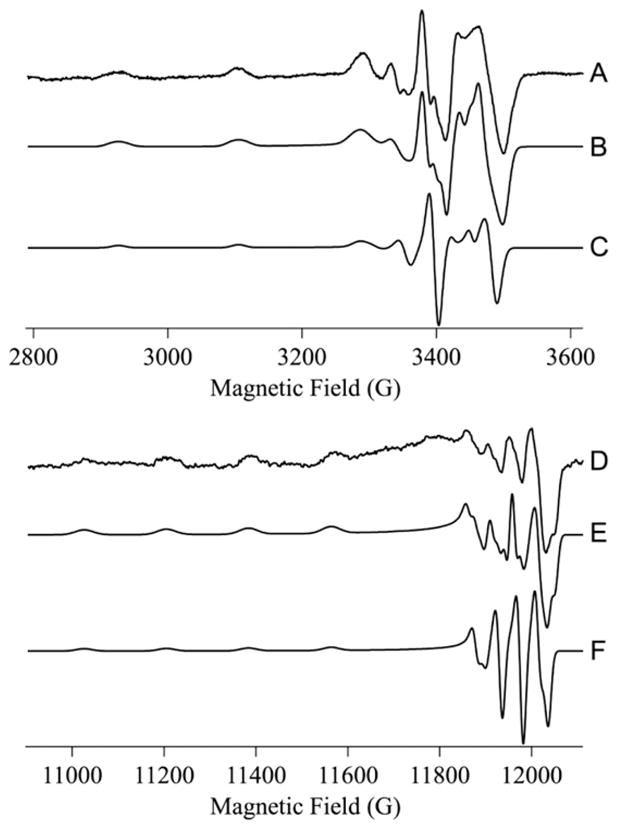
Simulations of the EPR spectra from Cu(II)-BsSCO at X- and Q-bands. (A) The 9.6 GHz EPR spectrum of the equilibrium species of BsSCO. (B) Simulation of A assuming g|| = 2.154, g⊥ = 2.033, Az,y,x Cu = 18.0, 4.5, 2.5 × 10−3 cm−1, and 1 × 14N with Az,y,xN = 1.1, 1.4, 1.1 × 10−3cm−1. (C) Computed spectrum using the same parameters as for B, except with no 14N superhyperfine interaction. (D) The 34 GHz EPR spectrum of the equilibrium species of BsSCO. (E) Simulation of D assuming g|| = 2.154, g⊥ = 2.034, Az,y,x Cu = 18.0, 5.0, 3.5 × 10−3 cm−1, and 1 × 14N with Az,y,xN = 1.1, 1.4, 1.1 × 10−3 cm−1. (F) Computed spectrum using the same parameters as for E, except with no 14N superhyperfine interaction. The slightly different parameters for Az,y,x Cu at the two frequencies likely indicates some non-coincidence of the g and A tensors.
3.2. Properties of the intermediate species
To gain more information on the nature of the intermediate species, the spectrum of the equilibrium species was subtracted from the species isolated by RFQ after 140 ms incubation of BsSCO with Cu(II). The difference spectrum (Fig. 1K) is distinct from the spectrum of CuCl2(aq) and represents a new intermediate species in the binding of Cu(II) by BsSCO. Computer simulation (Fig. 1L) returned values of g|| = 2.251, g⊥ = 2.049 and A|| Cu = 16.6 × 10−3 cm−1. These values clearly indicate the presence of at least two equatorially coordinated nitrogens, and argue strongly against equatorial sulfur coordination (Fig. 2). Nitrogen coordination cannot be detected directly from superhyperfine structure in the spectrum, but the inclusion of typical values for rhombically distorted axial (super)hyperfine couplings to the copper nucleus and to two nitrogens in the simulation of Fig. 1L shows that the shape and width of the g⊥ line is consistent with the presence of at least two nitrogens. The origin of the small feature in trace 1K at about 3360 G (geff ≈ 2.04) is unclear; the feature could be approximated by including a small axial copper quadrupolar component of 0.3–0.4 × 10−3 cm−1, but the fit is not sufficiently good to infer either imidazole or axial nitrogen coordination. In addition, this feature overlaps considerably with the g⊥ line of the CuCl2(aq.) signal. Further information on the nitrogen coordination of the intermediate species is expected to arise from electron spin echo envelope modulation and from very low frequency EPR studies [21,22].
The EPR spectra of the species trapped by RFQ are modeled using the three basis spectra, i.e. CuCl2(aq.), the BsSCO intermediate species, and the BsSCO equilibrium species (Fig. 1C–H). In each case, the best fit is achieved using a 70 ± 5% contribution (in terms of spins) from the BsSCO intermediate species, a 10 ± 1% contribution from the BsSCO equilibrium species, and a 20 ± 10% contribution from CuCl2(aq.). After 10–140 ms incubation with BsSCO, therefore, 70% of the added equimolar Cu(II) is in an intermediate state that is characterized by a higher equatorial nitrogen coordination number than the equilibrium state, and with no sulfur coordination.
4. Discussion
In these experiments the apo-form of reduced BsSCO was mixed with a CuCl2 solution in a 1:1 molar ratio. Although this reactant mixture is not ideal for kinetic analysis, that is not our primary concern here. Rather we kept to a low Cu(II):BsSCO ratio so as to minimize the contribution of “free Cu(II)” to the EPR spectrum. The EPR spectra observed at 10–140 ms under our experimental conditions has contributions from two spectral forms of Cu(II) in complex with BsSCO and this is consistent with our previous stopped-flow, transient absorption studies on the kinetics of Cu(II) binding to BsSCO [14]. There is also a small amount of the equilibrium copper bound species that is present in the sample before mixing. This species is hard to avoid during handling of the sample due to the extremely high affinity of reduced, apo-BsSCO for Cu(II) [12]. This equilibrium species is characterized by a superhyperfine splitting in the g⊥ region that could be very well modeled as being due to a single equatorially coordinated nitrogen atom, and that is presumably derived from the coordinated histidine residue (i.e., H135). Peisach and Blumberg analysis is entirely consistent with this conclusion, and also strongly suggests two equatorial coordinated sulfurs; there are two coordinated cysteines in BsSCO. Hence, the EPR spectroscopy of the equilibrium species of BsSCO faithfully predicts the salient features of the Cu(II) coordination sphere.
The second and more strongly contributing species is a new form. Although the difference spectrum that corresponds to the intermediate species exhibits poorly resolved structure in the g⊥ region that hints at coordinated nitrogen, that region is complicated by extensive overlap with strong features from the other two species contributing to the experimental spectrum. In addition, even small Cu quadrupolar couplings manifest themselves as small features in this region when included in simulations. This structure cannot, therefore, be confidently assumed to indicate nitrogen coupling. On the other hand, the Peisach and Blumberg analysis is unambiguous in identifying that the Cu(II) ion in the intermediate species is equatorially coordinated by at least two nitrogens and not at all by sulfur. This coordination environment is markedly different from both CuCl2(aq.) and the BsSCO equilibrium species.
It appears that Cu(II) is initially bound by nitrogens derived from BsSCO, most likely backbone peptide nitrogens, such as is observed at high Cu(II):protein ratios with the prion protein [22]. This initial complex then evolves to the final form with two sulfur atoms, from the two conserved cysteine residues, as inner sphere ligands. We propose that the initial BsSCO:Cu complex with nitrogen ligation serves to prevent copper catalyzed oxidation of the thiol-containing residues of BsSCO. Such oxidation is observed for thiol-based reagents [8] and for cysteine residues in other proteins [23]. Spontaneous conversion of the BsSCO–Cu(II) complex to oxidized BsSCO plus Cu(I) has been observed at high ionic strength with the native protein [6]. Redox conversion of the BsSCO–Cu(II) complex has also been observed for the H135A mutant of BsSCO at low ionic strength [24].
BsSCO binds copper(II) in a thermodynamically tight (KD ~3 pM) and kinetically inert (koff <10−5 s−1) complex. Progression to the equilibrium form takes only a couple of seconds at neutral pH with the isomerisation step occurring at a first order rate of 1.5 s−1 [14]. The thermodynamic stability is largely conferred by the energetics of the two-step binding process and slow kinetic exchange is determined by the off rate of the stable complex. At the observed formation rates if reduced BsSCO is exposed to Cu(II) in vivo the equilibrium species is likely to accumulate. One could view the equilibrium copper bound form of BsSCO as a potential trap and, if copper exchange is to occur, this trap must be escaped. If the rule of microscopic reversibility is applied then the intermediate characterized here represents a potential form of BsSCO that could be involved in metal exchange. However, conversion from the equilibrium, thiol-ligated form to the intermediate, nitrogen-ligated form would require an energetic input, as might be derived from the interaction of BsSCO with its target protein. The nitrogen-ligated intermediate does not have the stability of the final, thiol-ligated form, and yet does not undergo premature thiol oxidation as reported for the H135A mutant of BsSCO [24]. The initial interaction between BsSCO and copper via nitrogen ligation may be critical to maintain the reduced, functional state of BsSCO.
Acknowledgments
The work was supported by an NSERC (Canada) Grant to B.C.H. and by a Research for a Healthier Tomorrow/Advancing a Healthier Wisconsin grant to B.B.
Abbreviations
- SCO
accessory protein required for synthesis of cytochrome c oxidase
- BsSCO
SCO1 homolog from Bacillus subtilis
- EPR
electron paramagnetic resonance
- RFQ
rapid-freeze-quench
- ESEEM
electron spin echo envelope modulation
References
- 1.Glerum DM, Shtanko A, Tzagoloff A. SCO1 and SCO2 act as high copy suppressors of a mitochondrial copper recruitment defect in Saccharomyces cerevisiae. J Biol Chem. 1996;271:20531–20535. doi: 10.1074/jbc.271.34.20531. [DOI] [PubMed] [Google Scholar]
- 2.Mattatall NR, Jazairi J, Hill BC. Characterization of YpmQ, an accessory protein required for the expression of cytochrome c oxidase in Bacillus subtilis. J Biol Chem. 2000;275:28802–28809. doi: 10.1074/jbc.M002741200. [DOI] [PubMed] [Google Scholar]
- 3.Horng YC, Leary SC, Cobine PA, Young FB, George GN, Shoubridge EA, Winge DR. Human Sco1 and Sco2 function as copper-binding proteins. J Biol Chem. 2005;280:34113–34122. doi: 10.1074/jbc.M506801200. [DOI] [PubMed] [Google Scholar]
- 4.Ye Q, Imriskova-Sosova I, Hill BC, Jia Z. Identification of a disulfide switch in BsSco, a member of the Sco family of cytochrome c oxidase assembly proteins. Biochemistry. 2005;44:2934–2942. doi: 10.1021/bi0480537. [DOI] [PubMed] [Google Scholar]
- 5.Abriata LA, Banci L, Bertini I, Ciofi-Baffoni S, Gkazonis P, Spyroulias GA, Vila AJ, Wang S. Mechanism of Cu(A) assembly. Nat Chem Biol. 2008;4:599–601. doi: 10.1038/nchembio.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson DE, Hill BC. Stability of oxidized, reduced and copper bound forms of Bacillus subtilis. Sco Biochim Biophys Acta. 2009;1794:275–281. doi: 10.1016/j.bbapap.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Cavallini D, De Marco C, Dupre S, Rotilio G. The copper catalyzed oxidation of cysteine to cystine. Arch Biochem Biophys. 1969;130:354–361. doi: 10.1016/0003-9861(69)90044-7. [DOI] [PubMed] [Google Scholar]
- 8.Kachur AV, Koch CJ, Biaglow JE. Mechanism of copper-catalyzed autoxidation of cysteine. Free Radical Res. 1999;31:23–34. doi: 10.1080/10715769900300571. [DOI] [PubMed] [Google Scholar]
- 9.Mishra R, Mukhopadhyay S, Banerjee R. Reduction mechanism of a coordinated superoxide by thiols in acidic media. Dalton Trans. 2010;39:2692–2696. doi: 10.1039/b918582h. [DOI] [PubMed] [Google Scholar]
- 10.Kachur AV, Held KD, Koch CJ, Biaglow JE. Mechanism of production of hydroxyl radicals in the copper-catalyzed oxidation of dithiothreitol. Radiat Res. 1997;147:409–415. [PubMed] [Google Scholar]
- 11.Ramirez DC, Mejiba SE, Mason RP. Copper-catalyzed protein oxidation and its modulation by carbon dioxide: enhancement of protein radicals in cells. J Biol Chem. 2005;280:27402–27411. doi: 10.1074/jbc.M504241200. [DOI] [PubMed] [Google Scholar]
- 12.Imriskova-Sosova I, Andrews D, Yam K, Davidson D, Yachnin B, Hill BC. Characterization of the redox and metal binding activity of BsSco, a protein implicated in the assembly of cytochrome c oxidase. Biochemistry. 2005;44:16949–16956. doi: 10.1021/bi051343i. [DOI] [PubMed] [Google Scholar]
- 13.Andruzzi L, Nakano M, Nilges MJ, Blackburn NJ. Spectroscopic studies of metal binding and metal selectivity in Bacillus subtilis BSco, a homologue of the yeast mitochondrial protein Sco1p. J Am Chem Soc. 2005;127:16548–16558. doi: 10.1021/ja0529539. [DOI] [PubMed] [Google Scholar]
- 14.Cawthorn TR, Poulsen BE, Davidson DE, Andrews D, Hill BC. Probing the kinetics and thermodynamics of copper(II) binding to Bacillus subtilis Sco, a protein involved in the assembly of the Cu(A) center of cytochrome c oxidase. Biochemistry. 2009;48:4448–4454. doi: 10.1021/bi802288m. [DOI] [PubMed] [Google Scholar]
- 15.Garrity JD, Bennett B, Crowder MW. Direct evidence that the reaction intermediate of metallo-beta-lactamase L1 is metal bound. Biochemistry. 2005;44:1078–1087. doi: 10.1021/bi048385b. [DOI] [PubMed] [Google Scholar]
- 16.Hanson GR, Gates KE, Noble CJ, Mitchell A, Benson S, Griffin M, Burrage K. XSophe – Sophe – Xepr view a computer simulation software suite for the analysis of continuous wave EPR spectra. In: Shiotani M, Lund A, editors. EPR of Free Radicals in Solids: Trends in Methods and Applications. Kluwer Press; Netherlands: 2003. pp. 197–237. [Google Scholar]
- 17.Rist GH, Ammeter J, Günthard HH. Influence of the host lattice upon the EPR coupling parameters of copper-8-hydroxyquinolinate. J Chem Phys. 1968;49:2210–2217. [Google Scholar]
- 18.Rothenberger KS, Nilges MJ, Altman TE, Glab K, Belford RL, Froncisz W, Hyde JS. L-band parallel mode EPR. Measurement of quadrupole coupling through direct observation of secondary transitions. Chem Phys Lett. 1986;124:295–298. [Google Scholar]
- 19.Liczwek DL, Belford RL, Pilbrow JR, Hyde JS. Evaluation of copper nuclear quadrupolar coupling in thio complexes by completion of the coordination sphere. J Phys Chem. 1983;87:2509–2512. [Google Scholar]
- 20.Peisach J, Blumberg WE. Structural implications derived from the analysis of electron paramagnetic resonance spectra of natural and artificial copper proteins. Arch Biochem Biophys. 1974;165:691–708. doi: 10.1016/0003-9861(74)90298-7. [DOI] [PubMed] [Google Scholar]
- 21.Hyde JS, Bennett B, Walter ED, Millhauser GL, Sidabras JW, Antholine WE. EPR of Cu2+ prion protein constructs at 2 GHz using the g(perpendicular) region to characterize nitrogen ligation. Biophys J. 2009;96:3354–3362. doi: 10.1016/j.bpj.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chattopadhyay M, Walter ED, Newell DJ, Jackson PJ, Aronoff-Spencer E, Peisach J, Gerfen GJ, Bennett B, Antholine WE, Millhauser GL. The octarepeat domain of the prion protein binds Cu(II) with three distinct coordination modes at pH 7.4. J Am Chem Soc. 2005;127:12647–12656. doi: 10.1021/ja053254z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsui LI, Suzuki M, Hayashi N, Hu J, Van Eldik LJ, Titani K, Nishikimi M. Copper-dependent formation of disulfide-linked dimer of S100B protein. Arch Biochem Biophys. 2000;374:137–141. doi: 10.1006/abbi.1999.1595. [DOI] [PubMed] [Google Scholar]
- 24.Siluvai GS, Nakano MM, Mayfield M, Nilges MJ, Blackburn NJ. H135A controls the redox activity of the Sco copper center. Kinetic and spectroscopic studies of the His135Ala variant of Bacillus subtilis Sco. Biochemistry. 2009;48:12133–12144. doi: 10.1021/bi901480g. [DOI] [PMC free article] [PubMed] [Google Scholar]


