Table 1.
Nγ-Substituted pyrazolylthiazole analogs.
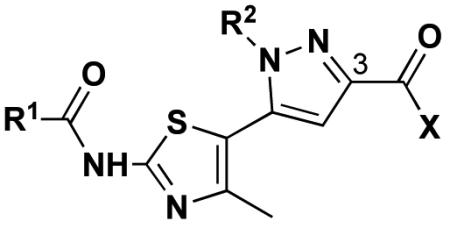
R1 = C(CH3)3, C6H5
R2 = CH2CH=CH2, C6H4(4-Br), CH2C6H5, (CH2)2OH
X = OEt, NHC6H4(4-OMe), NHCH2C6H5, NH(CH2)2OH, N(CH2CH2)2O, OH
| Compd | R1 | R2 | X |
|---|---|---|---|
| 9a | C(CH3)3 | CH2CH=CH2 | OEt |
| 9b | C(CH3)3 | C6H4(4-Br) | OEt |
| 9c | C(CH3)3 | CH2C6H5 | OEt |
| 9d | C(CH3)3 | CH2CH2OH | OEt |
| 9e | C6H5 | CH2CH=CH2 | OEt |
| 9f | C6H5 | C6H4(4-Br) | OEt |
| 9g | C6H5 | CH2C6H5 | OEt |
| 9h | C6H5 | CH2CH2OH | OEt |
| 11a | C(CH3)3 | CH2CH=CH2 | NHC6H4(4-OMe) |
| 11b | C(CH3)3 | CH2CH=CH2 | NHCH2C6H5 |
| 11c | C(CH3)3 | CH2CH=CH2 | N(CH2CH2)2O |
| 11d | C(CH3)3 | C6H4(4-Br) | NHC6H4(4-OMe) |
| 11e | C(CH3)3 | C6H4(4-Br) | NHCH2C6H5 |
| 11f | C(CH3)3 | C6H4(4-Br) | N(CH2CH2)2O |
| 11g | C(CH3)3 | CH2C6H5 | NHC6H4(4-OMe) |
| 11h | C(CH3)3 | CH2C6H5 | NHCH2C6H5 |
| 11i | C(CH3)3 | CH2C6H5 | N(CH2CH2)2O |
| 11j | C(CH3)3 | CH2CH2OH | NHC6H4(4-OMe) |
| 11k | C(CH3)3 | CH2CH2OH | NHCH2C6H5 |
| 11l | C(CH3)3 | CH2CH2OH | N(CH2CH2)2O |
| 11m | C6H5 | CH2CH=CH2 | NH(CH2)OH |
| 11n | C6H5 | C6H4(4-Br) | NH(CH2)OH |
| 11o | C6H5 | CH2C6H5 | NH(CH2)OH |
| 11p | C6H5 | CH2CH2OH | NH(CH2)OH |
| 11q | C6H5 | CH2CH=CH2 | NHC6H4(4-OMe) |
| 11r | C6H5 | C6H4(4-Br) | NHC6H4(4-OMe) |
| 11s | C6H5 | CH2C6H5 | NHC6H4(4-OMe) |
| 11t | C6H5 | CH2CH2OH | NHC6H4(4-OMe) |
| 11u | C6H5 | CH2CH=CH2 | N(CH2CH2)2O |
| 11v | C6H5 | C6H4(4-Br) | N(CH2CH2)2O |
| 11w | C6H5 | CH2C6H5 | N(CH2CH2)2O |
| 11x | C6H5 | CH2CH2OH | N(CH2CH2)2O |
| 12a | C(CH3)3 | CH2CH=CH2 | OH |
| 12b | C(CH3)3 | C6H4(4-Br) | OH |
| 12c | C(CH3)3 | CH2C6H5 | OH |
| 12d | C6H5 | CH2CH=CH2 | OH |
| 12e | C6H5 | C6H4(4-Br) | OH |
| 12f | C6H5 | CH2C6H5 | OH |
| 12g | C6H5 | CH2CH2OH | OH |
