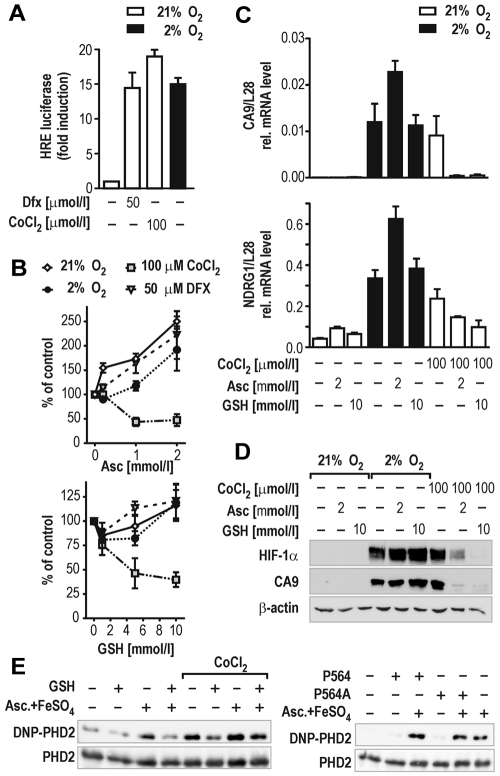
Figure 3.
GSH impairs HIF activation in cells. (A) Induction of HIF-dependent luciferase reporter gene activity in stably transfected HRG1 hepatoma cells by 2% O2, 50μM Dfx, or 100μM CoCl2 for 24 hours. (B) Effects of ascorbate (Asc, 0.2-2mM; top panel) or GSH (1-10mM; bottom panel) in combination with hypoxia, Dfx, or CoCl2 treatment on HIF-dependent luciferase activity relative to the protein concentration of the lysates. Shown are mean values ± SEM of 3 independent experiments normalized to the reporter activity in the absence of either ascorbate or GSH (control). (C) CA9 and NDRG1 HIF target gene mRNA levels in HRG1 cells after treatment with 2mM ascorbate or 10mM GSH combined with 2% O2 or 100μM CoCl2 for 24 hours. Shown are mean values ± SEM of 3 independent experiments relative to the mRNA content of ribosomal protein L28. (D) HIF-1α and CA9 protein levels in HRG1 cells after treatment with 2mM ascorbate or 10mM GSH combined with 2% O2 or 100μM CoCl2. (E) OxyBlot analyses of recombinant PHD2 protein carbonylation. GSH (5mM) reduced PHD2 carbonylation by either 2mM ascorbate/10μM FeSO4 or 100μM CoCl2 (left panel). PHD2 oxidation is independent of target hydroxylation as shown by using a wild-type or a Pro564Ala mutant HIF-1α hydroxyl-proline acceptor peptide in in vitro hydroxylation reactions (right panel).