Table 1.
Preparation of naphthocycloheptanones through couplling of δ,ε-unsaturated carbene complexes and 2-alkynylbenzoyl systems.
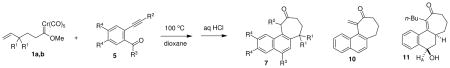 | |||||||
|---|---|---|---|---|---|---|---|
| entry | reactants | R1 | R2 | R3 | R4 | product | yield % |
| 1 | 1a + 5a | H | TMSa | Ph | H | 7a | 77 |
| 2 | 1a + 5b | H | TMSa | H | H | 7b | 73 |
| 3 | 1b + 5b | Me | TMSa | H | H | 7c | 75 |
| 4 | 1b + 5c | Me | H | H | H | 7c | 62 |
| 5b | 1a + 5d | H | n-Bu | H | H | 11 | 78 |
| 6c | 1a + 5e | H | CH2OAc | H | H | 10 | 74 |
| 7 | 1a + 5f | H | TMSa | H | OMe | 7g | 76 |
| 8 | 1b + 5g | Me | TMSa | Me | H | 7h | 80 |
R2 = H in 7 when R2 = TMS in 5.
The exclusive product was the alcohol derivative 11.
The exclusive product was elimination product 10.
